Biomed. adv. 1(1):27-33.
doi: 10.34172/bma.05
Original Article
Melatonin supplementation improves glycemic hemostasis, lipid profile, and adipokine concentrations of obese women: A double-blind randomized clinical trial
Naimeh Mesri Alamdari Conceptualization, Investigation, Visualization, Writing – original draft, 1, * 
Arvin Namazi Shabestari Writing – review & editing, 2 
Farzad Najafipour Methodology, Visualization, Writing – review & editing, 1 
Amirali Mirmazhari Data curation, Formal analysis, Methodology, Writing – review & editing, 3
Author information:
1Endocrine Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2College of Science, University of Tehran, Tehran, Iran
3Department of Laboratory Sciences, Faculty of Para Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Summary
Introduction:
Obesity and related diseases are an important universal public issue that harms man’s well-being. Recently, growing attention has been paid to the anti-obesity effect of melatonin. This study aims to assess melatonin’s impact on obesity-related factors including glycemic status, adipokines levels, lipid, and anthropometric indices in women who are obese and undergoing a calorie-restricted diet.
Methods:
In this double-blind placebo-controlled randomized clinical trial (RCT) study, 46 obese women were randomly assigned into either melatonin (6 g/d) or placebo (6 g/d) group and calorie-restricted diets for 40 days. Serum concentrations of fasting blood sugar (FBS), insulin, leptin, adiponectin, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c), triglycerides (TG), low-density lipoprotein cholesterol (LDL-c), insulin resistance as well as anthropometric indices were evaluated at the beginning and end of the intervention.
Findings:
Melatonin consumption grave rise a marked diminish in insulin (P=0.006) and HOMA-IR (P=0.001), which the between-group comparisons were substantial only for HOMA-IR (P=0.020) after adjusting for confounders. Adiponectin levels improved remarkably relative to the placebo (P=0.010). Lipid measures including TG, LDL-c, and HDL-c declined remarkably in the melatonin group post-intervention, whereas among-group percent changes were notable in HDL-c after adjusting for confounders (P=0.040). Notable variations were not observed in anthropometric indices in the melatonin group, compared to the placebo at the final.
Conclusion:
the present study, revealed that melatonin supplementation markedly improved glycemic indices, adiponectin, and lipid profile related to obesity.
Trial Registration:
Identifier: IRCT2012122411867N1; https://irct.behdasht.gov.ir/.
Keywords: Adiponectin, Insulin, Low-calorie diet, Melatonin, Obesity
Copyright and License Information
© 2024 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
The Research Vice Chancellor of Tabriz University of Medical Sciences, IRAN sponsored the study Grant number: 76542.
Introduction
It is known that; fatness is a challenging universal public health issue. It is estimated that almost 2.5 billion people are overweight and obese which includes one-third of the whole world population.1 In adults, the fatness rate was nearly 18.5% and 14% among women and men respectively in 2022.2 Obesity results in multiple complications, like type 2 diabetes mellitus (T2DM), cancers, lipid dysregulations, fatty liver disorder, and cardiovascular disorders which all of them cause complexity in obesity management.3
The augmented increment in obesity is to some extent explained by an imbalance between calorie intake and increased sedentary lifestyle, inappropriate food choices, low quality of sleep, and epigenetic susceptibility.4-6 White adipose tissue (WAT) as a critical endocrine organ discharges bioactive molecules including the adipokines regulate lipid metabolism, energy balance, insulin sensitivity, angiogenesis immunity, and inflammation.7,8
Melatonin (N-acetyl-5-methoxytryptamine) as an internal indoleamine hormone takes part in different physiologic procedures and is regulated through the hypothalamic suprachiasmatic nucleus (SCN). It is rhythmically produced in the pineal gland and does its role by melatonin receptor 1 (MT1) and melatonin receptor 2 (MT2), G-protein-coupled membrane receptors.9 Furthermore, melatonin has prominent impacts on the circadian rhythm, immunity, energy metabolism, and endocrine system.5,10
Numerous metabolic pathways in adipose tissue, like the induction and discouragement of adipokines, are controlled by circadian rhythm that is partly regulated by melatonin, which may act on WAT through MT1 and MT2 or using the sympathetic nervous system.3,11,12
Different animal studies emphasize the beneficial effects of melatonin in the obesity management.13-17 The investigations about melatonin’s effect on obesity in humans are scarce.18-20 The aims of this study are the evaluation of melatonin’s effect on the glycemic variables, adipokines level mediated in the regulation of energy, lipid profiles, and anthropometric indices of obese women under the low-calorie diet.
Methods
Study design and subjects
Obese subjects referred from outpatient clinics of Tabriz University of Medical Sciences, Tabriz, Iran were employed in the present double-blind placebo-controlled randomized clinical trial (RCT), carried out from February to July 2023. Eligible subjects were adults with body mass index (BMI) ≥ 30 kg/m2 and between 20 to 50 years, stable body weight within the past 6 months. Subjects were eliminated if they were pregnant or lactation period, menopause phase, smoking, consuming alcohol, hypertension, diabetes mellitus, consuming medicine that may affect lipid and glucose function like glucose-lowering, lipid-modifying drugs or kidney disorders, mental disease, using tranquilizers, contraceptives, anti-inflammatory drugs, taking any antioxidant supplements, anti-obesity drugs, multivitamins, in the last 3 months. Moreover, patients were excluded if less than 90% of their intervention were consumed. Patients completed the written informed consent at baseline. Personal features like demographic data were taken from all participants.
Sample size calculation
The mean ± standard deviation (SD) of high-density lipoprotein cholesterol (HDL-c)21 was applied for sample size computation. Considering the confidence interval (CI) of 95% and power of 90%, 21 subjects were calculated for each group. Supposing a 10% drop-out rate, the sample size for each group is considered 23.
Randomization, blinding, and intervention
The randomized block approach was used to allocate the obese subjects through Random Allocation Software (RAS) to melatonin and/or placebo arms (1:1) by research staff who were not involved in the procedure of the study. Randomization was conducted by block of size 2 for age and BMI. The assistant supplied the supplement and placebo tablets with a code consisting of three digits for any treatment. All the participants and research staff were blinded relative to the study arms.
The assignments of participants to each group were concealed from the research experts before the randomization.
Melatonin supplemented group (n = 23) took two melatonin tablets, (3 mg each; Nature Made, USA) once a day 2h before bedtime together with a calorie-restricted diet, while the placebo group (n = 23) took 2 placebo tablets, 3 mg each which resembled the melatonin tablets (encompass cellulose and starch) once a day 2 hours preceding bedtime for 40 days. Calorie-restricted diets were planned for subjects by an expert dietitian, based on the specific features of the subjects, considering a daily caloric limitation (500 kcal below the total energy expenditure measured by Mifflin Eq). Participants were controlled 3 times throughout the study for evaluation of any adverb effects of supplementation.
The supplement and placebo were provided every 2 weeks. Participants must return their unconsumed supplements and placebo every two weeks so the compliance rate can be calculated. Dietary recommendations were explained to the participants, and weight variations were evaluated every 2 weeks. It was requested by the participants to keep their ordinary lifestyles and follow the dietary suggestions.
Evaluation of anthropometric indices
Anthropometric measures were evaluated before and after intervention. Weight and height were assessed with minimal clothing and without shoes through a stadiometer to the nearest 100 g and 0.5 cm, respectively. Then, BMI was estimated as weight (kg) divided by height squared (m2). Waist circumference (WC) and hip circumference (HC) were determined using a measuring tape to the nearest 0.1 kg.
Laboratory measurements
After the 8–12 hour overnight fasting state, blood samples were gathered. The serum was detached and stocked at −80 °C until measurements. Fasting blood sugar (FBS), total cholesterol (TC), HDL-c, and TG through the colorimetric-enzymatic procedure by commercial kits (Pars-Azmoon Co, Tehran, Iran). Low-density lipoprotein cholesterol (LDL-c) was determined by Friedewald formula.22
Insulin was measured by the enzyme-linked immunosorbent assay (ELISA) procedure with the commercial kits (Crystal Day, Shanghai, China). The homeostatic model assessment for IR (HOMA-IR) was used as follows23: HOMA-IR = [fasting insulin (µIU/mL) × fasting glucose (mg/dL)] /405.
Study outcomes
The main outcomes of the study were glycemic indices, adipokines levels, and lipid profile variations. The secondary outcomes were anthropometric indices variations.
Statistical analysis
Data entry and analysis were done by SPSS Statistics software (IBM SPSS Statistics, Armonk, USA, version 26). The Kolmogorov–Smirnov examinations were applied to measure the distribution of continuous variables. The mean ± SD was used for numerical data and frequency (percentage) for categorical data. Between-group and within-group changes were computed by independent samples t-test and paired samples t-test respectively. Percentage changes were determined by the following equation:
[(after intervention measures – before intervention measures)/ before intervention measures] × 100 calculated and among-group variations were evaluated by the analysis of covariance (ANCOVA) exam which is adjusted for the confounding factors (i.e., baseline measures, energy intake, and BMI. The significance level was defined at a P value lower than 0.05.
Results
Of 46 obese patients, 44 subjects (22 subjects in each group) fulfilled the investigation. In the melatonin-supplemented group, one person used below 90% of the supplements, since using the tranquilizers as required medicine was eliminated. In the placebo arm, one person was eliminated since did not follow the weight loss diet program properly (Figure 1). The mean age of participants was 33.86 ± 6.94 years old in the melatonin arm and 34.86 ± 7.29 years old in the placebo arm. No remarkable variations were observed in demographic, and anthropometric variables of the study participants at baseline (P≥ 0.05). Indeed, melatonin supplementation for forty days resulted in a –3% decrement in BMI, while it declined to –2.5% in the placebo group relative to baseline values, which comparisons of percent changes between groups declare that there are no marked variations between studied arms (P = 0.130). Furthermore, the among-group comparison for weight, waist, and hip circumference changes declares that there is no marked discrepancy among groups for the mentioned anthropometric indices (P ≥ 0.05).
Figure 1.
Study flow diagram
.
Study flow diagram
As presented in Table 1, no marked variations related to glucose-related factors were observed among groups at the beginning of the study(P > 0.05). Melatonin supplementation declined the insulin levels (P = 0.006) and HOMA-IR (P = 0.001) significantly, while there were no remarkable variations in the placebo group following intervention in mentioned variables. The discrepancy in percentage changes of glucose-related variables among groups was notable just in HOMA-IR (18.24% vs 4.57% decrement in intervention and placebo arms respectively, P = 0.020) considering the role of the potential confounders (Figure 2A).
Table 1.
Glucose related and adipokines variables of the study participants throughout the study
|
Variables
|
Melatonin group (n=22)
|
P
value*
|
Placebo group (n=22)
|
P
value*
|
|
Before
|
After
|
Before
|
After
|
| FBS (mg/dL) |
98.17 ± 63.91 |
59.9 ± 13.86 |
141.0 |
74.24 ± 50.98 |
99.25 ± 45.99 |
592.0 |
| Insulin (μU/mL) |
70.4 ± 99.19 |
77.4 ± 53.17 |
006.0 |
89.5 ± 27.18 |
95.4 ± 31.17 |
091.0 |
| HOMA-IR |
56.1 ± 55.4 |
06.1 ± 72.3 |
001.0 |
54.1 ± 37.4 |
40.1 ± 17.4 |
120.0 |
| Adiponectin (ng/mL) |
45.1 ± 40.7 |
2.3 ± 59.9 |
001.0 > |
2.67 ± .599 |
41.2 ± 71.8 |
0.341 |
| Leptin (ng/mL) |
18.97 ± 94.46 |
16.18 ± 42.58 |
282.0 |
82.20 ± 66.48 |
40.15 ± 59.36 |
0.571 |
Mean (SD) are presented for data presentation.
∗P value for paired t test.
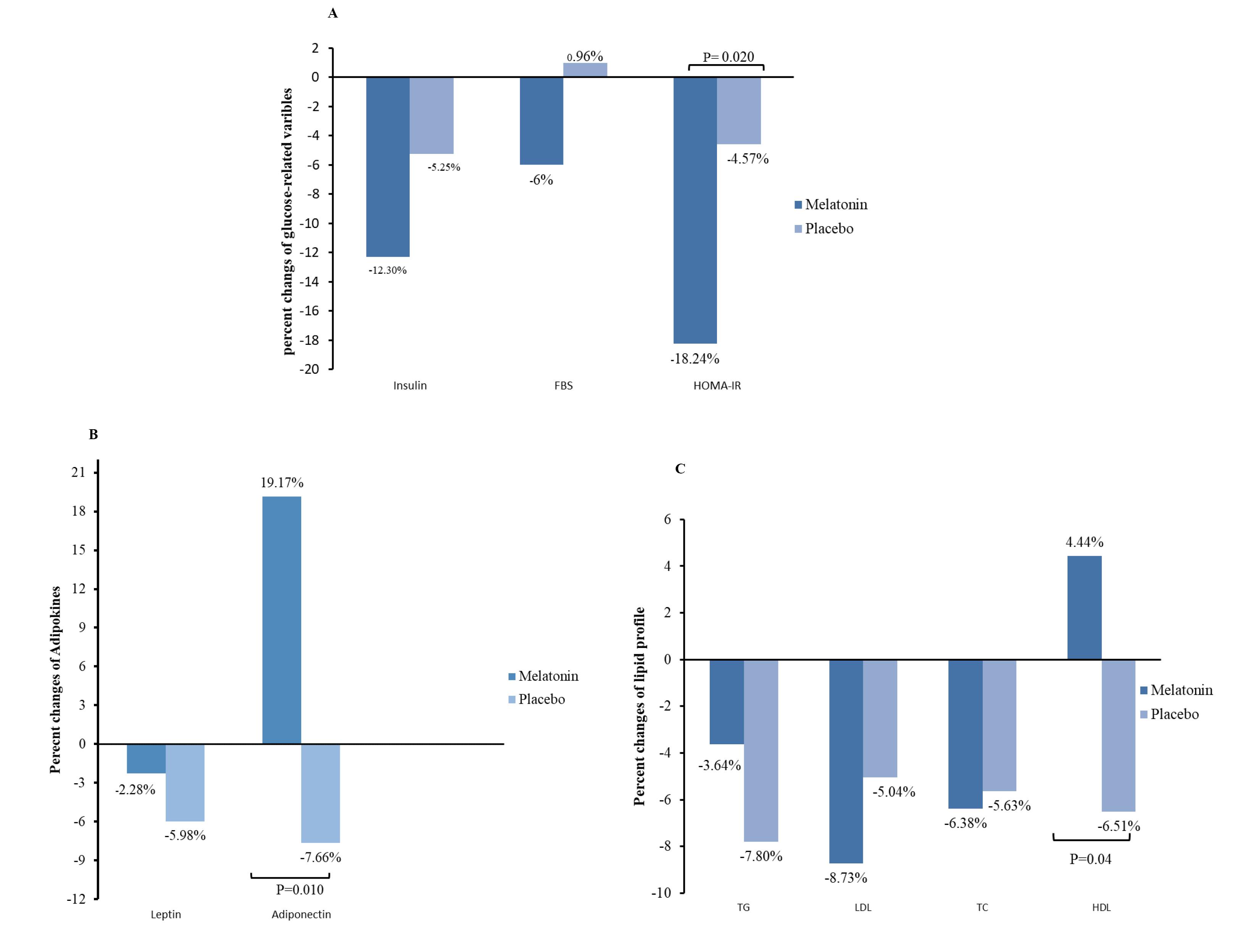
Figure 2.
Comparison of percentage changes of glucose related parameters(A), adipokines(B) and lipid profiles (C) between the two study groups. P < 0.05, by independent samples t-test
.
Comparison of percentage changes of glucose related parameters(A), adipokines(B) and lipid profiles (C) between the two study groups. P < 0.05, by independent samples t-test
There were no variations between groups in leptin and adiponectin concentrations at baseline(P > 0.05). However, after 40 days, adiponectin increased remarkably in the melatonin arm (P < 0.001), while it did not alter in the placebo arm. Leptin levels did not change significantly after 40 days relative to baseline values in both groups (Table 1). Indeed, following adjustments for confounders were done, adiponectin levels meliorated markedly in the melatonin arm relative to the placebo arm ( + 19.17% vs - 7.66%, P = 0.010) whereas leptin did not vary significantly between the two groups (Figure 2B).
Table 2 presents the evaluation of lipid profiles between the two studied groups. At baseline, there was no striking discrepancy between the studied arms considering the lipid profile (P > 0.05). Following the intervention, melatonin resulted in a marked decrement of serum LDL-c (P = 0.042) and TC (P = 0.040), meanwhile, the TG and HDL-c concentrations did not vary remarkably. Regarding the placebo arm, no remarkable alterations were noticed in lipid profile levels. Figure 2C shows the evaluations of percentage changes in lipid profile in two studied arms. Following adjustments were done for confounders, melatonin contributed to HDL-c levels augmentation in comparison to placebo (P= 0.040), while the percent changes in other lipid-related parameters were not significant between the two studied groups.
Table 2.
Lipid profiles of the study participants throughout the study
|
Variables
|
Melatonin group (n=22)
|
P
value*
|
Placebo group (n=22)
|
P
value*
|
|
Before
|
After
|
Before
|
After
|
| TG (mg/dL) |
57.62 ± 45.175 |
37.61 ± 05.169 |
461.0 |
57.56 ± 41.130 |
32.56 ± 23.120 |
0.110 |
| LDL-c (mg/dL) |
09.28 ± 05.113 |
45.27 ± 18.103 |
042.0 |
29.23 ± 65.124 |
.5425 ± 36.118 |
0.051 |
| TC (mg/dL) |
04.34 ± 95.194 |
68.34 ± 50.182 |
040.0 |
74.45 ± 55.202 |
.3743 ± 14.191 |
0.310 |
| HDL-c (mg/dL) |
68.8 ± 86.39 |
49.9 ± 63.41 |
153.0 |
46.8 ± 90.43 |
8.87 ± .0441 |
0.20 |
Mean (SD) are presented for data presentation.
∗P value for paired t test.
Discussion
Obesity, the pandemic health problem, is associated with complicated chronic disease. Although
modern clinical approaches to obesity management have evolved, the therapeutic challenge continues. Among numerous supplements already examined, the last investigations proposed that melatonin consumption might be an effective therapeutic perspective in obesity control.
This clinical trial assessed melatonin supplementation impacts on anthropometric indices, the glycemic, adipokines, and lipid profile of obese women undergoing a calorie-restricted diet. This investigation observed that melatonin administration in obese women for 40 days led to a noticeable promotion in cardiometabolic risk factors including HDL-c, insulin resistance, adiponectin, and insulin levels, with no remarkable effects on anthropometric indices like weight and BMI after adjusting for the confounders.
Increasing evidence suggests the promoting effects of melatonin consumption on insulin resistance and glucose-related factors. Our results showed a remarkable decrement in insulin concentrations and HOMA-IR, but no notable following adjustments were done for confounders which are consistent with former animal9,11,24 and clinical research.25
A late systematic review and meta-analysis of RCTs evaluated the melatonin supplementation impact and brought about a marked drop in FBS, insulin, and HOMA-IR.26 Furthermore, A recent RCT observed that melatonin supplementation during 12 weeks in patients with T2DM and coronary heart disease remarkably declined FBS, and serum insulin, and promoted the HOMA-IR20 The underlying mechanism of melatonin in amelioration of insulin sensitivity encompasses induction of β -cell regeneration, hepatic glycogen synthesis promotion which reduce hyperglycemia in an animal model.13,27,28 Furthermore, melatonin prevents insulin resistance by activating the cyclic adenosine monophosphate (cAMP)-response element binding protein (CREB)-peroxisome proliferator-activated receptor gamma coactivator 1-(PGC-1α) pathway.9 It also provokes the IRS1-PI3K-PKC route to improve glucose absorption in skeletal muscle.24
Adiponectin a hormone that is secreted from subcutaneous and visceral WAT depots involved in the regulation of lipid and glucose metabolism, ameliorates insulin sensitivity, modulates appetite and energy expenditure, and demonstrates anti-inflammatory and antiproliferative functions.8 Our results also showed the beneficial effects of 6 g/d of melatonin for 40 days on adiponectin levels without any significant changes in leptin after adjustment for potential confounders. Different animal models and clinical studies approved the melatonin’s effect on the treatment of circulating adiponectin.19,29,30 The underlying mechanism of melatonin action on adiponectin includes the effects of indoleamine function on adiponectin signaling pathways, the antioxidant and anti-inflammatory features of melatonin, progression in mitochondrial action, and effects on other adipokine concentrations.7 Melatonin administration decreases the circulating leptin levels in most animal models,29,30 however, the results are inconsistent in human studies.19,31 Leptin amount did not vary after a 40-day melatonin supplementation in the present study.
The present results also found that melatonin supplementation for 40 days declined TC and LDL-c levels while improving HDL-c. However, after confiders adjustments only improvements of HDL-c were significant. Experimental studies report the lowering properties of melatonin on serum TC concentrations prohibition of the absorption and biosynthesis of cholesterol and also increasing its catabolism.16,32 In agreement with our results, a new systematic review and meta-analysis of RCTs demonstrated the beneficial impacts of melatonin consumption on lipids at doses greater than 8 mg/d and long durations of more than 8 weeks including a notable reduction in TG, TC, and a small reduction in LDL-c and HDL-c.33 The lipid-promoting effects of melatonin arise from the promotion of innate cholesterol release through the production of bilirubin acid and the prevention of low-density lipoprotein receptor function. Moreover, enhancement of irisin levels and improvement fecal cholesterol repulsion are some pathways that melatonin acts.16,34,35
Growing evidence showed that melatonin supplementation decreased weight in animals.17,36-38 However, the clinical evidence reveals melatonin supplements did not have striking effects on weight and the results of various doses and times on weight are inconsistent.39-42 It was reported that melatonin is effective in weight reduction in those subjects with mental disease and consumed drugs led to adverse effects like weight variations. More research with various doses and lengths of study is requisite to approve this effect. The suggested mechanism for the obesity-modifying feature of melatonin is induction of lipolysis in adipocytes by up-regulation of the expression of hormone-sensitive lipase, adipocyte triglyceride lipase, and perilipin 1 via MT2, increasing cellular respiratory capacity through upregulation of the PGC-1, and transcription factor A mitochondrial expression and induction of the expression of thermogenic genes in adipocytes, as carnitine palmitoyl transferase and UCP3, which cause diffractions to beige phenotype formation.10,12,14
Strengths and limitations of the study
An important strength of the present work was the small omission rate, and great admission rate of the subjects to the supplements in both arms, considering possible confounders and using individualized dietary plans for weight reduction as an approved method in obesity combat. However, the restricted number of participants, the low study period, and the small melatonin dose were this investigation’s limitations.
Conclusion
Melatonin shows beneficial impacts on the improvement of adiponectin, lipid-related factors, insulin resistance, and obesity-related complications. The interactive mechanism of action of melatonin including the regulation of adipose tissue metabolism, and insulin resistance, may be involved in obesity management. Most importantly, more investigation with great supplement dose, prolonged study duration, and more extensive methods are needed to unravel the path in which melatonin takes part in the fatness procedure.
Competing Interests
The authors declare no conflict of interest.
Ethical Approval
The Ethics Committee of Tabriz University of Medical Sciences reviewed and approved the protocol (Ethical code: IR.TBZMED.REC: 924). Moreover, the investigation was carried out in accordance with the Helsinki Declaration of 1964, and its later amendments. All participants filled out the informed consent in the study. Additionally, it was registered in the Iranian Registry of Clinical Trials (identifier: IRCT2012122411867N1; http://www.irct.ir).
Acknowledgements
We sincerely wish to thank the study patients and researchers who participated and conducted the present study.
References
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384(9945):766-81. doi: 10.1016/s0140-6736(14)60460-8 [Crossref] [ Google Scholar]
- World Health Organization (WHO). Obesity and Overweight. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- Sheehan MT, Jensen MD. Metabolic complications of obesity Pathophysiologic considerations. Med Clin North Am 2000; 84(2):363-85. doi: 10.1016/s0025-7125(05)70226-1 [Crossref] [ Google Scholar]
- Lavie CJ, Laddu D, Arena R, Ortega FB, Alpert MA, Kushner RF. Healthy weight and obesity prevention: JACC health promotion series. J Am Coll Cardiol 2018; 72(13):1506-31. doi: 10.1016/j.jacc.2018.08.1037 [Crossref] [ Google Scholar]
- Huang Q, Ma C, Chen L, Luo D, Chen R, Liang F. Mechanistic insights into the interaction between transcription factors and epigenetic modifications and the contribution to the development of obesity. Front Endocrinol (Lausanne) 2018; 9:370. doi: 10.3389/fendo.2018.00370 [Crossref] [ Google Scholar]
- Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care 2011; 14(4):402-12. doi: 10.1097/MCO.0b013e3283479109 [Crossref] [ Google Scholar]
- Liu M, Liu F. Regulation of adiponectin multimerization, signaling and function. Best Pract Res Clin Endocrinol Metab 2014; 28(1):25-31. doi: 10.1016/j.beem.2013.06.003 [Crossref] [ Google Scholar]
- Szewczyk-Golec K, Woźniak A, Reiter RJ. Inter-relationships of the chronobiotic, melatonin, with leptin and adiponectin: implications for obesity. J Pineal Res 2015; 59(3):277-91. doi: 10.1111/jpi.12257 [Crossref] [ Google Scholar]
- Ha E, Yim SV, Chung JH, Yoon KS, Kang I, Cho YH. Melatonin stimulates glucose transport via insulin receptor substrate-1/phosphatidylinositol 3-kinase pathway in C2C12 murine skeletal muscle cells. J Pineal Res 2006; 41(1):67-72. doi: 10.1111/j.1600-079X.2006.00334.x [Crossref] [ Google Scholar]
- Yang W, Tang K, Wang Y, Zhang Y, Zan L. Melatonin promotes triacylglycerol accumulation via MT2 receptor during differentiation in bovine intramuscular preadipocytes. Sci Rep 2017; 7(1):15080. doi: 10.1038/s41598-017-12780-y [Crossref] [ Google Scholar]
- Chen J, Xia H, Zhang L, Zhang H, Wang D, Tao X. Protective effects of melatonin on sepsis-induced liver injury and dysregulation of gluconeogenesis in rats through activating SIRT1/STAT3 pathway. Biomed Pharmacother 2019; 117:109150. doi: 10.1016/j.biopha.2019.109150 [Crossref] [ Google Scholar]
- Pan S, Guo Y, Hong F, Xu P, Zhai Y. Therapeutic potential of melatonin in colorectal cancer: Focus on lipid metabolism and gut microbiota. Biochim Biophys Acta Mol Basis Dis 2022; 1868(1):166281. doi: 10.1016/j.bbadis.2021.166281 [Crossref] [ Google Scholar]
- Li T, Ni L, Zhao Z, Liu X, Lai Z, Di X. Melatonin attenuates smoking-induced hyperglycemia via preserving insulin secretion and hepatic glycogen synthesis in rats. J Pineal Res 2018; 64(4):e12475. doi: 10.1111/jpi.12475 [Crossref] [ Google Scholar]
- Liu K, Yu W, Wei W, Zhang X, Tian Y, Sherif M. Melatonin reduces intramuscular fat deposition by promoting lipolysis and increasing mitochondrial function. J Lipid Res 2019; 60(4):767-82. doi: 10.1194/jlr.M087619 [Crossref] [ Google Scholar]
- Stacchiotti A, Favero G, Giugno L, Golic I, Korac A, Rezzani R. Melatonin efficacy in obese leptin-deficient mice heart. Nutrients 2017; 9(12):1323. doi: 10.3390/nu9121323 [Crossref] [ Google Scholar]
- Tung YT, Chiang PC, Chen YL, Chien YW. Effects of melatonin on lipid metabolism and circulating irisin in Sprague-Dawley rats with diet-induced obesity. Molecules 2020; 25(15):3329. doi: 10.3390/molecules25153329 [Crossref] [ Google Scholar]
- Xu P, Wang J, Hong F, Wang S, Jin X, Xue T. Melatonin prevents obesity through modulation of gut microbiota in mice. J Pineal Res 2017; 62(4):e12399. doi: 10.1111/jpi.12399 [Crossref] [ Google Scholar]
- Mesri Alamdari N, Mahdavi R, Roshanravan N, Lotfi Yaghin N, Ostadrahimi AR, Faramarzi E. A double-blind, placebo-controlled trial related to the effects of melatonin on oxidative stress and inflammatory parameters of obese women. Horm Metab Res 2015; 47(7):504-8. doi: 10.1055/s-0034-1384587 [Crossref] [ Google Scholar]
- Szewczyk-Golec K, Rajewski P, Gackowski M, Mila-Kierzenkowska C, Wesołowski R, Sutkowy P. Melatonin supplementation lowers oxidative stress and regulates adipokines in obese patients on a calorie-restricted diet. Oxid Med Cell Longev 2017; 2017:8494107. doi: 10.1155/2017/8494107 [Crossref] [ Google Scholar]
- Raygan F, Ostadmohammadi V, Bahmani F, Reiter RJ, Asemi Z. Melatonin administration lowers biomarkers of oxidative stress and cardio-metabolic risk in type 2 diabetic patients with coronary heart disease: a randomized, double-blind, placebo-controlled trial. Clin Nutr 2019; 38(1):191-6. doi: 10.1016/j.clnu.2017.12.004 [Crossref] [ Google Scholar]
- Koziróg M, Poliwczak AR, Duchnowicz P, Koter-Michalak M, Sikora J, Broncel M. Melatonin treatment improves blood pressure, lipid profile, and parameters of oxidative stress in patients with metabolic syndrome. J Pineal Res 2011; 50(3):261-6. doi: 10.1111/j.1600-079X.2010.00835.x [Crossref] [ Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18(6):499-502. doi: 10.1093/clinchem/18.6.499 [Crossref] [ Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28(7):412-9. doi: 10.1007/bf00280883 [Crossref] [ Google Scholar]
- Teodoro BG, Baraldi FG, Sampaio IH, Bomfim LH, Queiroz AL, Passos MA. Melatonin prevents mitochondrial dysfunction and insulin resistance in rat skeletal muscle. J Pineal Res 2014; 57(2):155-67. doi: 10.1111/jpi.12157 [Crossref] [ Google Scholar]
- Cagnacci A, Arangino S, Renzi A, Paoletti AM, Melis GB, Cagnacci P. Influence of melatonin administration on glucose tolerance and insulin sensitivity of postmenopausal women. Clin Endocrinol (Oxf) 2001; 54(3):339-46. doi: 10.1046/j.1365-2265.2001.01232.x [Crossref] [ Google Scholar]
- Doosti-Irani A, Ostadmohammadi V, Mirhosseini N, Mansournia MA, Reiter RJ, Kashanian M. The effects of melatonin supplementation on glycemic control: a systematic review and meta-analysis of randomized controlled trials. Horm Metab Res 2018; 50(11):783-90. doi: 10.1055/a-0752-8462 [Crossref] [ Google Scholar]
- She M, Deng X, Guo Z, Laudon M, Hu Z, Liao D. NEU-P11, a novel melatonin agonist, inhibits weight gain and improves insulin sensitivity in high-fat/high-sucrose-fed rats. Pharmacol Res 2009; 59(4):248-53. doi: 10.1016/j.phrs.2009.01.005 [Crossref] [ Google Scholar]
- Kanter M, Uysal H, Karaca T, Sagmanligil HO. Depression of glucose levels and partial restoration of pancreatic beta-cell damage by melatonin in streptozotocin-induced diabetic rats. Arch Toxicol 2006; 80(6):362-9. doi: 10.1007/s00204-005-0055-z [Crossref] [ Google Scholar]
- Kitagawa A, Ohta Y, Ohashi K. Melatonin improves metabolic syndrome induced by high fructose intake in rats. J Pineal Res 2012; 52(4):403-13. doi: 10.1111/j.1600-079X.2011.00955.x [Crossref] [ Google Scholar]
- Nduhirabandi F, Huisamen B, Strijdom H, Blackhurst D, Lochner A. Short-term melatonin consumption protects the heart of obese rats independent of body weight change and visceral adiposity. J Pineal Res 2014; 57(3):317-32. doi: 10.1111/jpi.12171 [Crossref] [ Google Scholar]
- Gonciarz M, Bielański W, Partyka R, Brzozowski T, Konturek PC, Eszyk J. Plasma insulin, leptin, adiponectin, resistin, ghrelin, and melatonin in nonalcoholic steatohepatitis patients treated with melatonin. J Pineal Res 2013; 54(2):154-61. doi: 10.1111/j.1600-079X.2012.01023.x [Crossref] [ Google Scholar]
- da Silva Mendes de Farias T, Cruz MM, da Cunha de Sa RC, Severi I, Perugini J, Senzacqua M. Melatonin supplementation decreases hypertrophic obesity and inflammation induced by high-fat diet in mice. Front Endocrinol (Lausanne) 2019; 10:750. doi: 10.3389/fendo.2019.00750 [Crossref] [ Google Scholar]
- Mohammadi-Sartang M, Ghorbani M, Mazloom Z. Effects of melatonin supplementation on blood lipid concentrations: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr 2018; 37(6 Pt A):1943-54. doi: 10.1016/j.clnu.2017.11.003 [Crossref] [ Google Scholar]
- Chan TY, Tang PL. Effect of melatonin on the maintenance of cholesterol homeostasis in the rat. Endocr Res 1995; 21(3):681-96. doi: 10.1080/07435809509030483 [Crossref] [ Google Scholar]
- Müller-Wieland D, Behnke B, Koopmann K, Krone W. Melatonin inhibits LDL receptor activity and cholesterol synthesis in freshly isolated human mononuclear leukocytes. Biochem Biophys Res Commun 1994; 203(1):416-21. doi: 10.1006/bbrc.1994.2198 [Crossref] [ Google Scholar]
- Onaolapo AY, Adebisi EO, Adeleye AE, Olofinnade AT, Onaolapo OJ. Dietary melatonin protects against behavioural, metabolic, oxidative, and organ morphological changes in mice that are fed high-fat, high- sugar diet. Endocr Metab Immune Disord Drug Targets 2020; 20(4):570-83. doi: 10.2174/1871530319666191009161228 [Crossref] [ Google Scholar]
- Mendes C, Gomes G, Belpiede LT, do Carmo Buonfiglio D, Motta-Teixeira LC, Amaral FG. The effects of melatonin daily supplementation to aged rats on the ability to withstand cold, thermoregulation and body weight. Life Sci 2021; 265:118769. doi: 10.1016/j.lfs.2020.118769 [Crossref] [ Google Scholar]
- Wang L, McFadden JW, Yang G, Zhu H, Lian H, Fu T. Effect of melatonin on visceral fat deposition, lipid metabolism and hepatic lipo-metabolic gene expression in male rats. J Anim Physiol Anim Nutr (Berl) 2021; 105(4):787-96. doi: 10.1111/jpn.13497 [Crossref] [ Google Scholar]
- Bahrami M, Cheraghpour M, Jafarirad S, Alavinejad P, Asadi F, Hekmatdoost A. The effect of melatonin on treatment of patients with non-alcoholic fatty liver disease: a randomized double-blind clinical trial. Complement Ther Med 2020; 52:102452. doi: 10.1016/j.ctim.2020.102452 [Crossref] [ Google Scholar]
- Marqueze EC, Nogueira LF, Vetter C, Skene DJ, Cipolla-Neto J, Moreno CRC. Exogenous melatonin decreases circadian misalignment and body weight among early types. J Pineal Res 2021; 71(2):e12750. doi: 10.1111/jpi.12750 [Crossref] [ Google Scholar]
- Treister-Goltzman Y, Peleg R. Melatonin and the health of menopausal women: a systematic review. J Pineal Res 2021; 71(2):e12743. doi: 10.1111/jpi.12743 [Crossref] [ Google Scholar]
- Mohammadi S, Rastmanesh R, Jahangir F, Amiri Z, Djafarian K, Mohsenpour MA. Melatonin supplementation and anthropometric indices: a randomized double-blind controlled clinical trial. Biomed Res Int 2021; 2021:3502325. doi: 10.1155/2021/3502325 [Crossref] [ Google Scholar]