Biomed adv. 2(2):61-76.
doi: 10.34172/bma.17
Systematic Review
Assessment of cardiac electrical activity in patients with polycystic ovary syndrome: A systematic review and meta-analysis
Seyedeh-Tarlan Mirzohreh Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Writing – original draft, 1, * 
Fariba Heidari Data curation, Formal analysis, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing, 2 
Author information:
1Department of Internal Medicine, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
2Department of Community and Family Medicine, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Summary
Polycystic ovary syndrome (PCOS) is a common endocrine disorder associated with metabolic disturbances, including insulin resistance and an increased risk of cardiovascular complications. This systematic review and meta-analysis, conducted following PRISMA guidelines, compared cardiac electrical activity in PCOS patients versus healthy controls. Databases (PubMed, Scopus, Web of Science, Cochrane) were searched using Joanna Briggs Institute (JBI) appraisal tools. Sixteen studies met inclusion criteria, with data categorized into atrial (P-wave) and ventricular (QT interval) electrical activity. Meta-analysis using RevMan and Comprehensive Meta-Analysis software revealed significant differences in atrial conduction. PCOS patients exhibited prolonged Pmax (mean difference=7.49; 95% CI [0.36, 14.63], P=0.04) and increased P dispersion (MD=10.74; 95% CI [5.96, 15.51], P<0.0001) compared to controls, while Pmin was shorter (MD=-2.23; 95% CI [-4.38, -0.08], P=0.04). For ventricular activity, only QTc interval was significantly shorter in PCOS patients (MD=-21.62; 95% CI [-37.70, -5.54], P=0.008), with no other QT abnormalities detected. These findings suggest that PCOS is associated with delayed atrial conduction, potentially increasing susceptibility to atrial arrhythmias, while ventricular repolarization remains largely unaffected. The study highlights a possible cardiac electrophysiological alteration in PCOS, emphasizing the need for closer cardiovascular monitoring in these patients. Further research should explore the long-term implications of these ECG changes on arrhythmia risk and cardiovascular outcomes in PCOS.
Keywords: Polycystic ovary syndrome, Cardiac electrical activity, Meta-analysis, Systematic review
Copyright and License Information
© 2025 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
There was no corporate or governmental funding for the present study.
Introduction
Polycystic ovary syndrome (PCOS), an intricate genetic condition, is the most prevalent heterogeneous syndrome of clinical and biochemical endocrine disorder among women of reproductive age.1 The foremost phenotype characteristics of women with PCOS are androgen excess, insulin resistance (IR), hypothalamic-pituitary-ovarian axis dysfunction, and deranged adipokines secretion from the adipose tissue.2
In addition to the fact that PCOS affects fertility and obstetrical conditions of the patients, it brings substantial health outcomes for women, impairing life quality.3 Since it is associated with other correlated lifestyle diseases, this syndrome presents significant metabolic and cardiovascular morbidities.4 Women with PCOS are highly susceptible to every component of metabolic syndrome, including dyslipidemia, type 2 diabetes, and hypertension.5-8 It is ascertained that metabolic syndrome indicates a condition of IR.9 Increased insulin level is crucial in promoting theca cells producing excess androgen and forming hyperandrogenism in women with PCOS.10 Hence, the co-existence of metabolic syndrome with PCOS can lead to greater existing cardiometabolic risk.
Recent studies distinctly claimed that there are significant cardiac conduction system abnormalities in PCOS patients, which can contribute to higher risks for cardiovascular diseases.11 Many studies have revealed that metabolic syndrome and its components can lead to electrocardiographic (ECG) abnormalities, such as longer P-wave duration and prolonged QT interval.12
These ECG abnormalities reflect pathologic changes in cardiac electric conduction 12. In clinical practice, atrial fibrillation (AF) is the most prevalent arrhythmia resulting from an abnormal cardiac conduction system. This abnormality can originate from increased sympathetic activity, high oxidation levels, ischemia, stress, and systemic inflammation.13-15 It has also been demonstrated that IR is capable of atrial remodeling and is associated with atrial arrhythmogenesis and AF even before diabetes develops.16 Moreover, gonadal steroids have been found to influence cardiac autonomic function and ion interactions and may lead to cardiac arrhythmias.17 Some studies were conducted to find possible p-wave and QT abnormalities and changes in the atrium and cardiac conduction system in PCOS patients.11,18-24
However, the actual relationship between PCOS and cardiac arrhythmia has remained controversial. In this systematic and meta-analysis review, we aim to assess the cardiac electrical activity to determine whether there is a possible elevated risk for cardiac arrhythmias in PCOS.
Methods
This systematic review was prepared using PRISMA reporting guidelines for systematic reviews25 and an a priori protocol registered with PROSPERO, CRD4202233705. During the conduct of the evaluation, we considered the following inclusion criteria.
Eligibility criteria
The overall inclusion criteria for the meta-analysis were as follows: (1) an original research article, (2) a study conducted in case-control, cross-sectional or observational (prospective or retrospective) cohort designs, (3) a study involved clinically and/or para-clinically and/or laboratory diagnosed PCOS, (4) a study reported mean and standard deviation (SD) of at least one of the ECG parameters including p-wave or QT indices or any other parameters measuring cardiac electrical activity other than ECG, (5) a study surveyed the relevant indices both in PCOS participants and control group, (6) a study excluded patients with known cardiovascular disease, thyroid disease, neoplasms, pregnancy or breast-feeding, smoking, chronic alcohol consumption, diabetes mellitus, hypertension, and renal impairment, (7) a study involved adult women in their reproductive age with or without PCOS, (8) no limitation for including articles published in languages other than English will be pursued.
The overall exclusion criteria for the meta-analysis were as follows: (1) abstracts, case reports, case series, any reviews, editorials, and practice guidelines, (2) a study involving menopausal and postmenopausal women with and without PCOS, (3) a study used data reporting forms like median value and interquartile range, (4) a study assessed the cardiac electrical activity in participant only diagnosed with metabolic-syndrome not specifically with PCOS.
Information sources
To identify the studies, we searched the following electronic databases until January 2023: PubMed, Scopus, Web of Science, and Cochrane, with no restrictions on time and language. We also performed a manual search through references in the found articles.
Search strategy
The search strategy of PubMed was to combine (((((((((((((((((((((“Electrophysiologic Techniques, Cardiac”[Mesh]) OR (“Electrocardiography”[Mesh])) OR (“electric* activity*”)) OR (electric*)) OR (“Heart Conduction System”[Mesh])) OR ((“Cardiac Conduction System Disease”[Mesh])) OR (electromechanical)) OR (“conduction delay”)) OR (“Arrhythmias, Cardiac”[Mesh])) OR (“p wave”)) OR (“p-wave”)) OR (QT*)) OR (“P dispersion”)) OR (“QT dispersion”)) OR (QRS)) OR (“T wave”)) OR (“T-wave”)) OR (atrial)) OR (ventricular)) OR (“left atrium”)) OR (“Atrial Fibrillation”[Mesh])) AND ((“Polycystic Ovary Syndrome”[Mesh]). The search strategy for Scopus, Web of Science, and the Cochrane Library is similar to what we used for searching PubMed (Table S1, Supplementary file 1). Two independent investigators scanned all the studies’ titles and abstracts to select applicable studies. In addition, two investigators manually screened reference lists from systematic reviews and selected studies independently to ensure all relevant studies have been included in this study.
Study selection
All records from the systematic search in the electronic database and reference lists of selected records were evaluated by two authors independently following the eligibility criteria mentioned above. After strict selection and evaluation, we collected the data from the records as follows: ECG indices, study design, numbers of PCOS cases and control group involved, published language, and baseline characteristics, including age, body mass index (BMI), blood pressure, heart rate, waist to hip ratio (WHR), relevant hormone and lipid profile. We categorized the data into two subgroups to assess the cardiac electrical activity in two forms of atrial and ventricular electrical activity in PCOS cases (Table 1).
Table 1.
Summary of studies included in the meta-analysis
|
First author and year of publication
|
Study design
|
Language of publication
|
PCOS cases
|
Controls
|
Electrocardiography indices
|
Baseline characteristics
|
|
Subgroup 1: Atrial electrical activity and PCOS
|
| Akdag et al, 201526 |
Cross-sectional |
English |
82 |
74 |
P max, P min, P dispersion |
Age, BMI, heart rate, SBP, DBP WHR, FBS, total cholesterol, TG, LDL, HDL, testosterone, estradiol, FSH, LH |
| Bayir et al, 201623 |
Cross-sectional |
English |
40 |
20 |
P max, P min, P dispersion, Lateral PA, Septal PA, Tricuspid PA |
Age, BMI, heart rate, FBS, total cholesterol |
| Erdogan et al, 201322 |
Case-control |
English |
40 |
46 |
P max, P min, P dispersion |
Age, BMI, WHR, heart rate, SBP, FBS, TG, LDL, HDL, testosterone, HOMA-IR, fasting insulin |
| Gazi et al, 201527 |
Case-control |
English |
48 |
38 |
P max, P min, P dispersion, Lateral tricuspid PA |
Age, BMI, heart rate, SBP, DBP, WHR, FBS, total cholesterol, TG, LDL, HDL, estradiol |
| Tasolar et al, 201428 |
Case-control |
English |
25 |
25 |
P max, P min, P dispersion, Lateral PA, Septal PA, Tricuspid PA |
Age, BMI, heart rate, SBP, DBP, FBS, Total Cholesterol, TG, LDL, HDL, testosterone, estradiol, HOMA-IR, fasting insulin |
| Tasolar et al, 201428 |
Case-control |
English |
25 |
25 |
P max, P min, P dispersion, Lateral PA, Septal PA, Tricuspid PA |
Age, BMI, heart rate, SBP, DBP, FBS, total cholesterol, TG, LDL, HDL, testosterone, estradiol, HOMA-IR, fasting insulin |
| Zehir et al, 201424 |
Case-control |
English |
51 |
48 |
P max, P min, P dispersion, Lateral PA, Septal PA, Tricuspid PA, Lateral tricuspid PA |
Age, BMI, heart rate, FBS, Total Cholesterol, TG, LDL, HDL, testosterone, estradiol, HOMA-IR, FSH, LH, fasting insulin |
|
Subgroup 2: Ventricular electrical activity and PCOS
|
| Akdag et al, 201526 |
Cross-sectional |
English |
82 |
74 |
Mean QTc, QT max, QT min, QTc dispersion, QTc max, QTc min, |
Age, BMI, heart rate, SBP, DBP, WHR, FBS, testosterone, estradiol, cholesterol, TG, LDL, HDL |
| Alpaslan et al, 200219 |
Cross-sectional |
English |
36 |
36 |
QT dispersion, QT max, QT min, QTc max, QTc min |
Age, BMI, heart rate, SBP, DBP, FBS, total cholesterol, TG, LDL |
| Balamurugan et al, 201620 |
Cross-sectional |
English |
24 |
24 |
Mean QTc, QT dispersion, QT max, QT min, QTc dispersion, QTc max, QTc min |
Age, BMI, SBP, DBP |
| Çakir et al, 201329 |
Case-control |
Turkish |
28 |
35 |
QT dispersion, QT max, QT min, QTc dispersion, QTc max, QTc min |
Age, BMI, heart rate, FBS, fasting insulin, insulin, HOMA-IR, testosterone, estradiol, cholesterol, TG, LDL, HDL, |
| Gateva et al, 201230 |
Cross-sectional |
English |
82 |
125 |
Mean QTc |
Age, BMI, heart rate, SBP, DBP, WHR, FBS, Insulin, HOMA-IR, total cholesterol, TG, LDL, HDL |
| Gazi et al, 2013 31 |
Cross-sectional |
English |
25 |
22 |
Mean QTc, QT max, QT min, QTc max, QTc min |
Age, heart rate, FBS, testosterone, estradiol, total cholesterol, HDL |
| Huang et al, 201011 |
Cross-sectional |
English |
24 |
12 |
Mean QTc, QT dispersion, QTc dispersion |
Age, BMI, heart rate, FBS, fasting insulin, testosterone |
| Karaagac et al, 201532 |
Cross-sectional |
Turkish |
36 |
31 |
Mean QTc, QT max |
Age, heart rate, SBP, DBP, FBS, testosterone, total cholesterol, TG, LDL, HDL |
| Meden-Vrtovec et al, 200733 |
Case-control |
English |
61 |
61 |
Mean QTc |
Age, insulin, testosterone |
| Orio et al, 200718 |
Case-control |
English |
50 |
50 |
QT dispersion, QT max, QT min, QTc dispersion, QTc max, QTc min |
Age, BMI, WHR, heart rate, SBP, DBP, FBS, insulin, HOMA-IR, testosterone, estradiol, total cholesterol, TG, LDL, HDL |
| Vrtovec et al, 200834 |
Cross-sectional |
English |
119 |
64 |
Mean QTc |
Heart rate, BMI, SBP, DBP, insulin, testosterone, total cholesterol |
Data extraction
Two reviewers did data extraction. Data were extracted from each subgroup and collected using Microsoft Excel® sheets. Paper IDs were extracted, including first author, year of publication, study population, and the number of PCOS patients and control groups. We extracted ECG indices and baseline characteristics and added them to the relevant subgroups as follows:
Subgroup 1: Atrial electrical activity and PCOS
ECG parameters: (1) P max, the longest atrial conduction time measured on any of the 12 ECG leads, (2) P min, the shortest atrial conduction time (3) and P dispersion is defined as the difference between P max and P min, (4) Atrial electromechanical coupling (PA) duration, the time interval from the beginning of P-wave on surface ECG to the onset of the late diastolic wave including (1) Lateral PA which is obtained from the lateral mitral annulus, (2) Septal PA is which obtained from the septal mitral annulus, and (3) Tricuspid PA which is obtained from the tricuspid annulus, and (4) Lateral tricuspid PA which is obtained from the lateral tricuspid annulus.
Subgroup 2: Ventricular electrical activity and PCOS
ECG parameters: (1) QT max, the most prolonged QT interval measured on any of the 12 ECG leads, (2) QT min, the shortest QT interval, (3) QT dispersion (QTd.QT dis), the difference between QT max and QT min, (4) Corrected QT (QTc), QT interval.square root of the RR interval and (5) similarly, corrected QT dispersion (QTcd or QTc dis) the difference between QTc max and QTc min.
Risk of bias assessment
Two individuals independently appraised the quality of the eligible studies before inclusion in the review using appraisal instruments from the Joanna Briggs Institute (JBI) for cross-sectional and case-control studies and other comparative studies. After the appraisal, studies that did not meet the methodological criteria were excluded, and reasons for their exclusion are provided in (Table S2).
Outcome quality assessment
The certainty of overall evidence was assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method.35 The assessment of evidence certainty for individual outcomes relied on five distinct criteria: (1) limitations of the study design; (2) consistency of results; (3) directness; (4) precision; and (5) potential for publication bias. A decrement of one level in certainty was implemented for each unfulfilled criterion.
Synthesis methods
We analyzed the data using RevMan software (version 5.3) with the random effect model and Comprehensive Meta-Analysis software (version 2). Statistical heterogeneity for each pooled estimate was calculated using Cochran’s chi-squared test and presented with the I2 statistic. The odds ratio (OR) and mean differences (absolute difference between the mean value in PCOS cases and control group. PCOS - Control) pool the data with 95% confidence intervals (CIs). Publication bias was visually assessed using funnel plots for the overall analysis of all included studies. P values of < 0.05 were considered to be statistically significant.
Results
Study selection
The study flowchart is shown in Figure 1; our search strategy revealed 877 records. After removing duplications, 579 studies went through title assessment. Of these, 107 reports were eligible for abstract review. After surveying abstracts, 22 studies met the inclusion and exclusion criteria and were perused for full text. Finally, 16 studies were qualified to be included in this systematic review and meta-analysis.
Figure 1.
Flow diagram of study selection
.
Flow diagram of study selection
Study characteristics
Sixteen original research articles were retrieved. Of these, nine studies were of a cross-sectional design, and the remaining studies were case-control. Two studies were published in Turkish,29,32 and the rest were redacted in English. All the studies were conducted in young patients newly diagnosed with PCOS with no medical history of any other health conditions, including cardiovascular disease, thyroid disease, neoplasms, pregnancy or breastfeeding, smoking, chronic alcohol consumption, diabetes mellitus, hypertension, and renal impairment. We divided the articles into two separate subgroups: (1) Atrial electrical activity and PCOS, (2) Ventricular electrical activity and PCOS, in terms of ECG parameters and baseline characteristics shown in Table 1. Among the studies of subgroup 1, all of them reported three P wave indices, including P max, P min, and P dispersion. Four studies also reported atrial electromechanical measures, including lateral PA and septal PA. Among studies of subgroup 2, three articles reported only mean QTc, and the rest reported at least two indices of the QT parameter. Most studies reported baseline characteristics, including age, BMI, systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate, and lipid and hormone profile of both PCOS cases and controls. Table 1 details the characteristics of all studies, including ECG measures, baseline characteristics, and involved population of PCOS and controls of each study.
Risk of bias assessment of included studies
Among sixteen studies included in this review, nine articles11,19,20,23,26,30-32,34 reported on the findings of a cross-sectional study design (Table 2). Seven articles18,24,22,27-29,33 reported on the findings of a case-control study design (Table 3). Overall, seven included articles scored 8.8, and two20,34 scored 6.8 using the scoring method for measuring the methodological qualities of cross-sectionals. Six articles scored 9.10 and one33 scored 7.10 using the scoring method for measuring the methodological qualities of case controls.
Table 2.
Summary score for methodological quality of analytic cross-sectional studies
|
Study ID
|
Q1
|
Q2
|
Q3
|
Q4
|
Q5
|
Q6
|
Q7
|
Q8
|
Total of “yes”
scores
|
| Akdag et al, 2015 26 |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
8 |
| Gazi et al, 201331 |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
8 |
| Vrtovec et al, 2008 34 |
Y |
Y |
Y |
Y |
NA |
NA |
Y |
Y |
6 |
| Alpaslan et al, 200219 |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
8 |
| Balamurugan et al, 2016 20 |
Y |
Y |
Y |
Y |
U |
N |
Y |
Y |
6 |
| Huang et al, 2010 11 |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
8 |
| Bayır et al, 201623 |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
8 |
| Gateva et al, 201130 |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
8 |
| Karaagac et al, 2015 32 |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
8 |
NB: Y = Yes, N = No, U = Unclear, NA = Not Applicable)
Q1. Were the criteria for inclusion in the sample clearly defined?
Q2. Were the study subjects and the setting described in detail?
Q3. Was the exposure measured in a valid and reliable way?
Q4. Were objective, standard criteria used for measurement of the condition?
Q5. Were confounding factors identified?
Q6. Were strategies to deal with confounding factors stated?
Q7. Were the outcomes measured in a valid and reliable way?
Q8. Was appropriate statistical analysis used?
Table 3.
Summary score for methodological quality of analytic case-control studies
|
Study ID
|
Q1
|
Q2
|
Q3
|
Q4
|
Q5
|
Q6
|
Q7
|
Q8
|
Q9
|
Q10
|
Total of “yes” scores
|
| Orio et al, 2007 18 |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
NA |
Y |
9 |
| Meden-Vrtovec et al, 2007 33 |
Y |
Y |
Y |
Y |
Y |
U |
U |
Y |
NA |
Y |
7 |
| Çakir et al, 201329 |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
NA |
Y |
9 |
| Zehir et al, 2014 24 |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
NA |
Y |
9 |
| Gazi et al, 201527 |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
NA |
Y |
9 |
| Tasolar et al, 201428 |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
NA |
Y |
9 |
| Erdogan et al, 2013 22 |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
NA |
Y |
9 |
(NB: Y = Yes, N = No, U = Unclear, NA = Not Applicable).
Q1. Were the groups comparable other than the presence of disease in cases or the absence of disease in controls?
Q2. Were cases and controls matched appropriately?
Q3. Were the same criteria used for identification of cases and controls?
Q4. Was exposure measured in a standard, valid and reliable way?
Q5. Was exposure measured in the same way for cases and controls?
Q6. Were confounding factors identified?
Q7. Were strategies to deal with confounding factors stated?
Q8. Were outcomes assessed in a standard, valid and reliable way for cases and controls?
Q9. Was the exposure period of interest long enough to be meaningful?
Q10. Was appropriate statistical analysis used?
GRADE assessment of outcomes
The GRADE assessment (Table 4) reveals that the certainty of evidence for outcomes related to atrial electrical activity and QT parameters predominantly ranges from Low to Moderate. Atrial electrical activity metrics (e.g., Pmax, PWD, Lateral PA) generally demonstrated Low certainty due to high heterogeneity, moderate risk of bias, or imprecision (confidence intervals crossing zero). Exceptions include P min and Mean QTc, which showed Moderate certainty. Among QT parameters, most outcomes (e.g., QT dispersion, QTc max) had Moderate certainty, while QTc min stood out with High certainty. Key limitations across studies included high inconsistency (heterogeneity), risk of bias in specific trials (e.g., Tasolar et al28), and imprecision from wide confidence intervals. Notably, Lateral Tricuspid PA had Very Low certainty due to an extremely limited number of studies. These findings underscore the need for further high-quality research to strengthen evidence confidence, particularly for outcomes with substantial methodological limitations.
Table 4.
GRADE assessment of outcomes
|
Assessment
|
Summary of findings
|
|
|
Risk of bias
|
Limitation
|
Inconsistency
|
Indirectness
|
Imprecision
|
Considerations
|
No Studies
|
MD (95% CI)
|
Certainty of Evidence
|
|
Atrial electrical activity
|
| Pmax |
Moderate1 |
Low |
High2 |
Low |
Moderate7 |
None |
7 |
7.49 [0.36, 14.63] |
ꚚꚚOO
Low |
| Pmin |
Low |
Low |
Moderate3 |
Low |
Low |
None |
7 |
-2.23 [-4.38, -0.08] |
ꚚꚚꚚO
Moderate |
| PWD |
Moderate1 |
Low |
High2 |
Low |
Low |
None |
7 |
10.74 [5.96, 15.51] |
ꚚꚚOO
Low |
| Lateral PA |
Moderate1 |
Moderate4 |
High2 |
Low |
Low |
None |
4 |
16.24 [6.29, 26.19] |
ꚚꚚOO
Low |
| Septal PA |
Moderate1 |
Moderate4 |
High2 |
Low |
Low |
None |
4 |
10.76 [1.88, 19.65] |
ꚚꚚOO
Low |
| Tricuspid PA |
Moderate5 |
Moderate4 |
High2 |
Low |
Moderate7 |
None |
4 |
3.11 [−1.40, 7.61] |
ꚚꚚOO
Low |
| Lateral tricuspid PA |
High6 |
High6 |
High2 |
Low |
Moderate7 |
None |
2 |
11.54 [-4.43, 27.52] |
ꚚOOO
Very Low |
|
Ventricular Electrical Activity |
| Mean QTc |
Moderate8 |
Low |
High2 |
Low |
Low |
None |
8 |
-21.62 [-37.70, -5.54] |
ꚚꚚꚚO
Moderate |
| QT dispersion |
Moderate8 |
Low |
High2 |
Low |
Moderate7 |
None |
6 |
2.39 [−3.17, 7.95] |
ꚚꚚꚚO
Moderate |
| QT max |
Low |
Low |
Low |
Low |
Moderate7 |
None |
7 |
-0.79 [-7.38, 5.80] |
ꚚꚚꚚO
Moderate |
| QT min |
High9 |
Low |
High2 |
Low |
Moderate7 |
None |
6 |
-6.70 [-16.71, 3.32] |
ꚚꚚꚚO
Moderate |
| QTc min |
Low |
Low |
Low |
Low |
Moderate7 |
None |
6 |
3.49 [-3.97, 10.96] |
ꚚꚚꚚꚚ
High |
| QTc max |
Low |
Low |
Moderate3 |
Low |
Moderate7 |
None |
6 |
-2.21 [−7.37, 2.96] |
ꚚꚚꚚO
Moderate |
1 The study conducted by Tasolar et al28 was found to have a high risk of bias, due to the different range of results reported.
2 The level of heterogeneity is high.
3 The level of heterogeneity is high.
4 The number of studies is low.
5 The study conducted by Zehir et al24 was found to have a high risk of bias, due to the different range of results reported.
6 The number of studies is very low.
7 Confidence interval includes 0.
8 The studies by Meden-Vrtovec et al33 and Vrtovec et al34 were have a high risk of bias, due to the different range of results reported, excluding them did not change the significance and direction of results.
9 The studies by Meden-Vrtovec et al33 was found to have a high risk of bias, due to the different range of results reported and excluding this study did not change the insignificance and direction of results.
Results of syntheses
We analyzed ECG measures in the form of 2 separate subgroups featuring P wave and QT indices. The clinical characteristics of each subgroup were analyzed separately. Table 5 represents result of ECG synthesis.
Table 5.
Electrocardiographic characteristics of included studies
|
Characteristics of studies
|
Number of studies
|
Number of PCOS
|
Number of controls
|
Mean difference (95% Cl)
|
P
value
|
Heterogeneity
|
|
I2
|
P
value
|
|
Subgroup 1: Atrial electrical activity and PCOS
|
| P max |
7 |
311 |
276 |
7.49 [0.36, 14.63] |
0.04 |
96% |
< 0.00001 |
| P min |
7 |
311 |
276 |
-2.23 [-4.38, -0.08] |
0.04 |
71% |
0.002 |
| PWD |
7 |
311 |
276 |
10.74 [5.96, 15.51] |
< 0.0001 |
95% |
< 0.00001 |
| Lateral PA |
4 |
141 |
118 |
16.24 [6.29, 26.19] |
0.001 |
98% |
< 0.00001 |
| Septal PA |
4 |
141 |
118 |
10.76 [1.88, 19.65] |
0.02 |
98% |
< 0.00001 |
| Tricuspid PA |
4 |
141 |
118 |
3.11 [−1.40, 7.61] |
0.18 |
95% |
< 0.00001 |
| Lateral tricuspid PA |
2 |
99 |
89 |
11.54 [-4.43, 27.52] |
0.16 |
98% |
< 0.00001 |
|
Subgroup 2: Ventricular electrical activity and PCOS
|
| Mean QTc |
8 |
511 |
416 |
-21.62 [-37.70, -5.54] |
0.008 |
91% |
< 0.00001 |
| QT dispersion |
6 |
244 |
231 |
2.39 [−3.17, 7.95] |
0.40 |
77% |
0.0005 |
| QT max |
7 |
281 |
272 |
-0.79 [-7.38, 5.80] |
0.81 |
37% |
0.14 |
| QT min |
6 |
245 |
241 |
-6.70 [-16.71, 3.32] |
0.19 |
75% |
0.001 |
| QTc dispersion |
5 |
162 |
157 |
0.58 [-2.21, 3.38] |
0.68 |
0% |
0.97 |
| QTc max |
6 |
245 |
241 |
3.49 [-3.97, 10.96] |
0.36 |
49% |
0.08 |
| QTc min |
6 |
245 |
241 |
-2.21 [−7.37, 2.96] |
0.40 |
16% |
0.31 |
Subgroup 1: Atrial electrical activity and PCOS
Among ECG analysis, Pmax, P dispersion were significantly longer in PCOS patients than in controls with effect size of 7.49 (95% CI [0.36, 14.63], p = 0.04) and 10.74 (95% CI [5.96, 15.51], P < 0.0001), respectively (Figure 2). Pmin was significantly shorter in PCOS cases compared to controls with an effect size of -2.23 (95% CI [-4.38, -0.08], P = 0.04) (Figure 2). It was revealed that lateral and septal PA were significantly longer in PCOS cases compared to controls with effect sizes of 16.24 (95% CI [6.29, 26.19], P = 0.001) and 10.76 (95% CI [1.88, 19.55], P = 0.02), respectively(Figure 3). Among baseline characteristics, heart rate tended to be higher in PCOS cases compared to controls, with an effect size of 1.62 (95% CI [0.07, 3.17], P = 0.04) (Table 6). PCOS cases were revealed to have higher BMI and WHR compared to the control group, which indicate obesity state in PCOS with effect size of 2.48 (95% CI [2.00, 2.95], P < 0.00001] and 0.03 (95% CI [0.01, 0.04], P < 0.00001), respectively. Serum testosterone, fasting insulin level and HOMA-IR were significantly higher in PCOS cases than control group with effect sizes of 30.43 (95% CI [24.89, 35.97], P < 0.00001), 5.24 (95% CI [0.49, 9.98], P < 0.00001) and 1.31 (95% CI [0.39, 2.23], P = 0.005) respectively. There were no other significant differences in baseline characteristics between PCOS cases and the control group (Table 6).
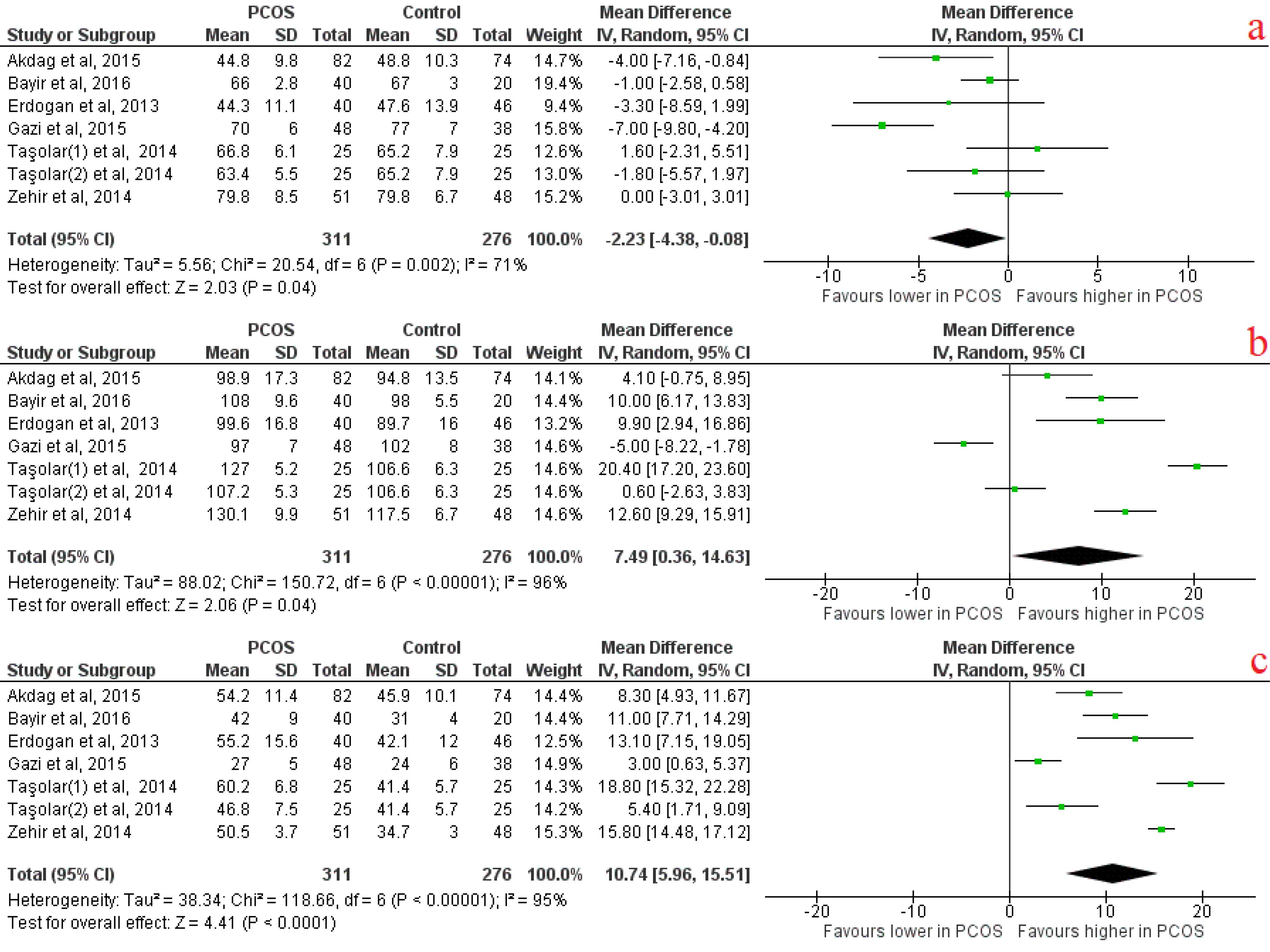
Figure 2.
Atrial electrical activity forest plots indicating A) P minimum, B) P maximum, C) P dispersion
.
Atrial electrical activity forest plots indicating A) P minimum, B) P maximum, C) P dispersion
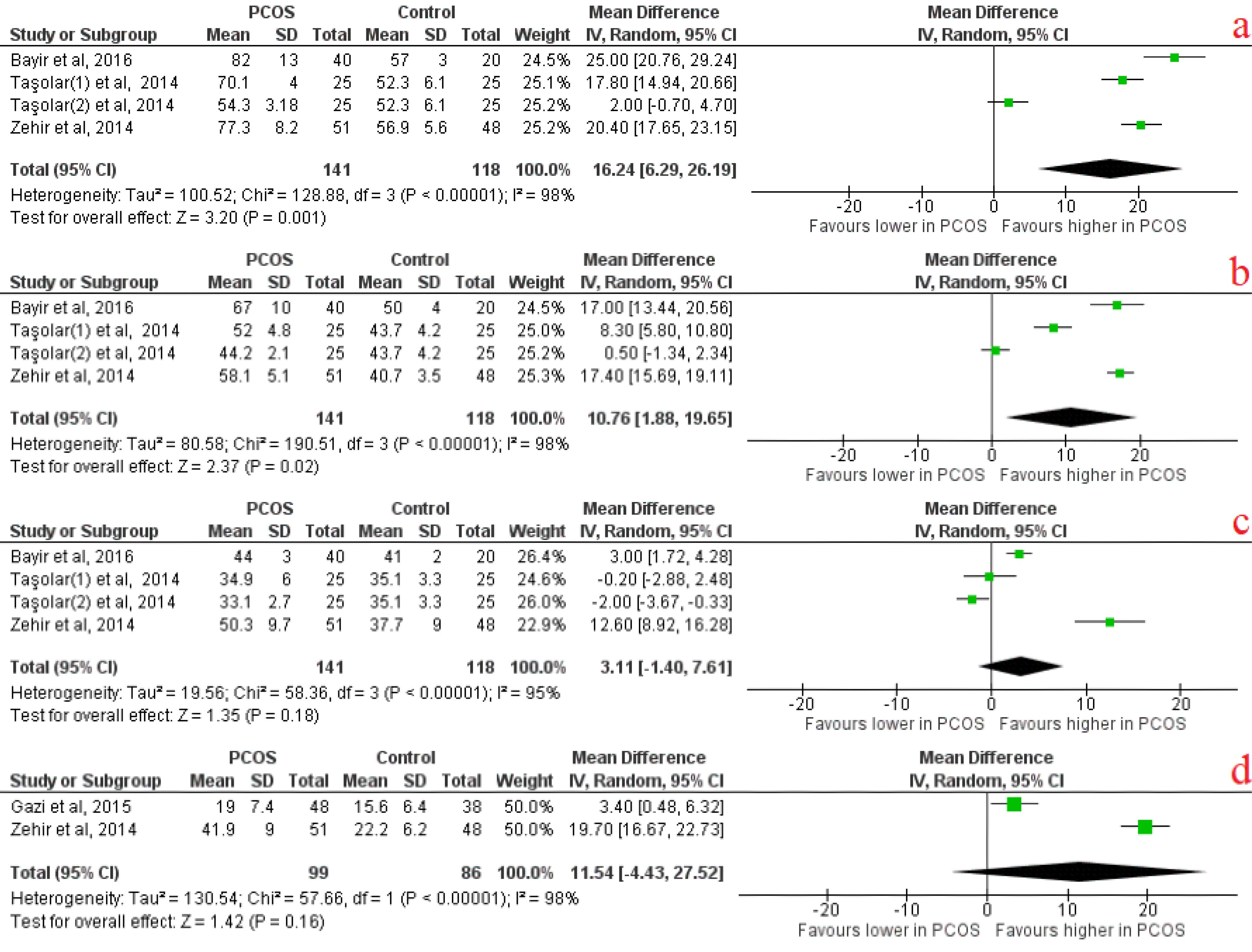
Figure 3.
Atrial electrical activity forest plots indicating: A) lateral PA, B) Tricuspid PA, C) Septal PA, D) Lateral tricuspid PA
.
Atrial electrical activity forest plots indicating: A) lateral PA, B) Tricuspid PA, C) Septal PA, D) Lateral tricuspid PA
Table 6.
Baseline characteristics of atrial and ventricular electrical activity and PCOS subgroups
|
Characteristics of studies
|
Number of studies
|
Number of PCOS
|
Number of controls
|
Mean difference (95% Cl)
|
P
value
|
Heterogeneity
|
|
I2
|
P
value
|
|
Subgroup 1: Atrial electrical activity Sand PCOS
|
| Age |
7 |
311 |
276 |
-0.94 [-2.45, 0.58] |
0.23 |
71% |
0.002 |
| BMI |
7 |
311 |
276 |
2.48 [2.00, 2.95] |
< 0.00001 |
98% |
< 0.00001 |
| WHR |
3 |
170 |
158 |
0.03 [0.01, 0.04] |
< 0.00001 |
0% |
0.64 |
| Heart rate |
7 |
311 |
276 |
1.62 [0.07, 3.17] |
0.04 |
0% |
0.50 |
| SBP |
5 |
220 |
208 |
1.13 [-0.59, 2.86] |
0.20 |
63% |
0.03 |
| DBP |
4 |
180 |
162 |
0.41 [-1.82, 5.65] |
0.72 |
38% |
0.18 |
| Total cholesterol |
6 |
271 |
230 |
5.36 [-9.19, 19.92] |
0.47 |
93% |
< 0.00001 |
| HDL |
6 |
271 |
256 |
-2.21 [-5.65, 1.22] |
0.21 |
84% |
< 0.00001 |
| LDL |
6 |
271 |
256 |
5.77 [-4.90, 46.44] |
0.29 |
89% |
< 0.00001 |
| Triglyceride |
6 |
271 |
265 |
12.65 [-10.32, 35.62] |
0.28 |
92% |
< 0.00001 |
| FBS |
7 |
311 |
276 |
0.91 [ -1.82, 3.64] |
0.51 |
86% |
< 0.00001 |
| Fasting insulin |
4 |
141 |
144 |
5.24 [0.49, 9.98] |
0.03 |
99% |
< 0.00001 |
| HOMA-IR |
4 |
141 |
144 |
1.31 [0.39, 2.23] |
0.005 |
98% |
< 0.00001 |
| Testosterone |
5 |
183 |
172 |
30.43 [24.89, 35.97] |
< 0.00001 |
75% |
0.008 |
| Estradiol |
5 |
231 |
210 |
-13.27 [-36.76, 10.22] |
0.27 |
99% |
< 0.00001 |
| FSH |
2 |
133 |
122 |
-0.60 [-1.58, 0.38] |
0.23 |
89% |
0.002 |
| LH |
2 |
133 |
122 |
0.17 [-0.41, 0.76] |
0.56 |
0% |
0.47 |
|
Subgroup 2: Ventricular electrical activity and PCOS
|
| Age |
11 |
625 |
537 |
-0.43 [-0.98, 0.11] |
0.12 |
2% |
0.43 |
| BMI |
9 |
481 |
451 |
0.16 [-0.59, 0.91] |
0.68 |
43% |
0.08 |
| WHR |
3 |
214 |
249 |
0.08 [-0.04, 0.2] |
0.19 |
99% |
< 0.00001 |
| Heart rate |
9 |
482 |
449 |
-0.17 [-2.15, 1.81] |
0.86 |
54% |
0.03 |
| SBP |
7 |
429 |
404 |
0.60 [-1.37, 2.57] |
0.55 |
0% |
0.56 |
| DBP |
7 |
429 |
404 |
1.28 [-0.13, 2.70] |
0.07 |
39% |
0.13 |
| Total cholesterol |
8 |
458 |
437 |
3.57 [-0.86, 7.99] |
0.11 |
0% |
0.92 |
| HDL |
7 |
339 |
373 |
-1.60 [-3.30, 0.10] |
0.07 |
0% |
0.69 |
| LDL |
6 |
314 |
351 |
4.13 [0.01, 8.25] |
0.05 |
0% |
0.46 |
| Triglyceride |
6 |
314 |
351 |
-0.02 [-8.20, 8.15] |
1.00 |
14% |
0.32 |
| FBS |
8 |
363 |
385 |
1.91 [0.38, 3.44] |
0.01 |
0% |
0.59 |
| Fasting insulin |
2 |
52 |
47 |
4.01 [0.67, 7.36] |
0.02 |
0% |
< 0.80 |
| Insulin |
5 |
370 |
303 |
5.95 [3.29, 8.61] |
< 0.0001 |
82% |
0.0002 |
| HOMA-IR |
3 |
160 |
210 |
1.25 [0.00, 2.51] |
0.05 |
89% |
< 0.0001 |
| Testosterone |
8 |
483 |
352 |
38.57 [21.19, 55.96] |
< 0.0001 |
96% |
< 0.00001 |
| Estradiol |
4 |
185 |
181 |
1.23 [-1.30, 3.76] |
0.34 |
0% |
0.59 |
| FSH |
3 |
160 |
159 |
-0.38 [-1.37, 0.61] |
0.45 |
86% |
0.0010 |
| LH |
3 |
150 |
159 |
5.57 [-4.15, 15.29] |
0.26 |
99% |
< 0.00001 |
Subgroup 2: Ventricular electrical activity and PCOS
It was revealed that mean QTc was significantly lower in PCOS patients compared to the control group, with an effect size of -21.62 (95% CI [-37.70, -5.54], P = 0.008). There were no other significant differences in QT indices (Figure 4). There was a significant difference in FBS levels (effect size of 1.91(95% CI [0.38, 3.44], P = 0.01), serum fasting insulin (effect size of 4.01(95% CI [ 0.67, 7.36], P = 0.02), serum insulin (effect size of 6.37(95% CI [3.49, 9.25], P < 0.0001) and serum testosterone (effect size of 38.57 (95% CI [21.19, 55.96], P < 0.0001) between PCOS cases and controls (Table 6).
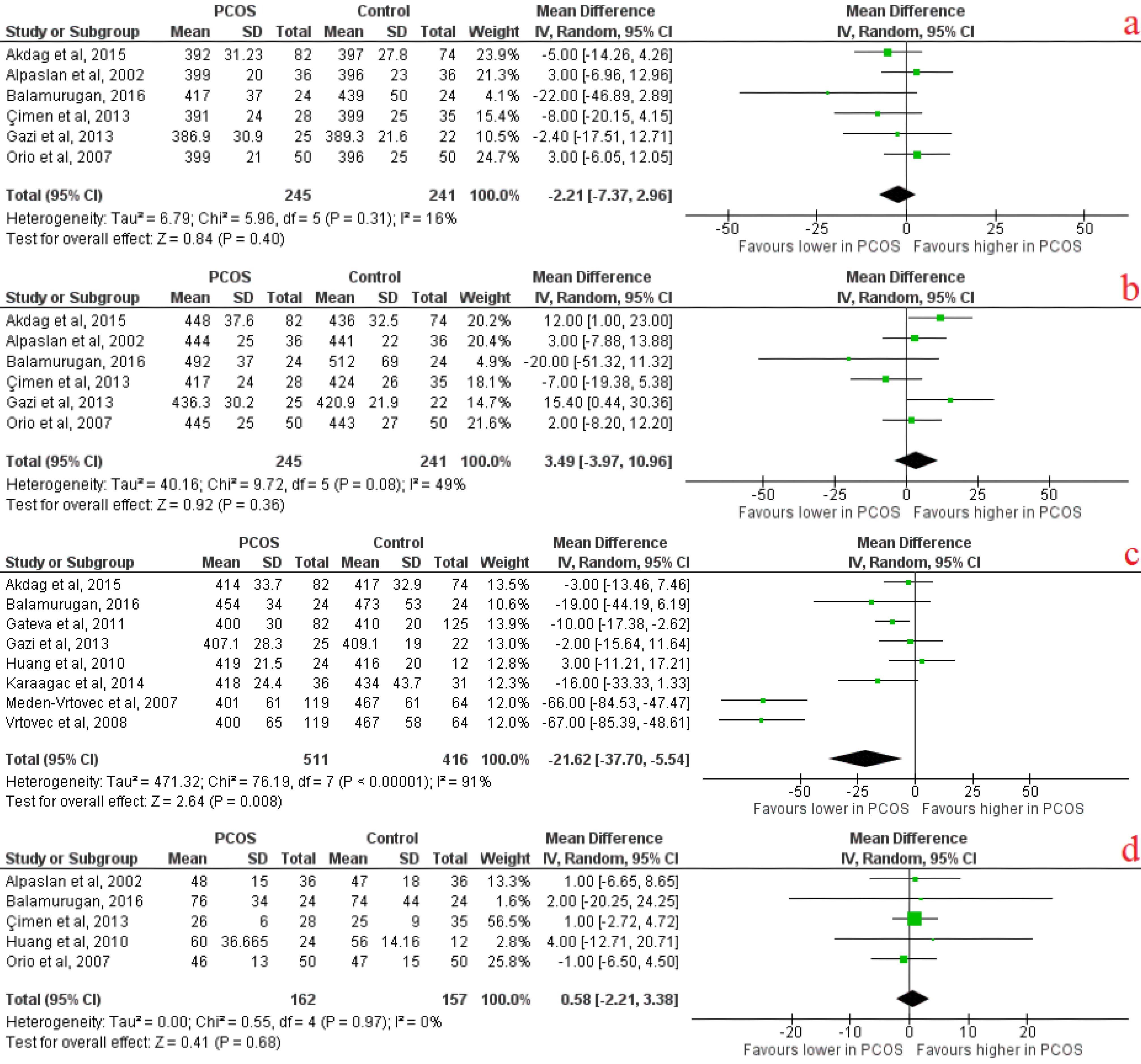
Figure 4.
Ventricular electrical activity forest plots indicating A) QTc minimum, B) QTc maximum, C) Mean QTc, D) QTc dispersion
.
Ventricular electrical activity forest plots indicating A) QTc minimum, B) QTc maximum, C) Mean QTc, D) QTc dispersion
Discussion
Atrial electrical activity and PCOS
Atrial remodeling is any atrium changes recognized by poor cellular coupling, inflammation, and fibrosis. Consequently, slowed electric conduction resulting from atrial remodeling develops an arrhythmic condition called AF.36 AF has been discovered as the most prevalent clinically substantial cardiac arrhythmia causing morbidities and mortalities. It is associated with a 5-fold elevated risk for stroke.37,38 It has been demonstrated that low-grade inflammation is signified in PCOS pathogenesis39. Results from a national Danish registry cohort study conducted by Oliver-Williams et al.40 Of 6149 (10.2%) patients with PCOS with a median follow-up of 8.9 years, 138 (0.2%) women developed AF. They reported that women with PCOS were at twice the risk of AF. Inflammation can modify atrial structure and electrophysiology, leading to structural and electrical remodeling, enhancing the vulnerability to AF.41
Furthermore, IR is shown to cause discordant autonomic nerve function, microvascular changes, and impaired blood glucose levels. Mentioned conditions can lead to myocardial fibrosis as a remodeling change that can cause atrial enlargement, leading to a prolonged conduction time.39 Moreover, elevated testosterone levels, a prominent diagnostic feature of PCOS, have increased the risk for AF and ischemic stroke.42,43
The meta-analysis of this study revealed that maximum p-wave duration (Pmax) was significantly higher in PCOS patients than in controls. Following this finding, Akdag et al,26 Zehir et al,24 Bayir et al,23 and Erdogan et al22 have reported significant longer Pmax in PCOS patients compared to controls; however, the absolute mean values of Pmax in the PCOS population of both included studies and this meta-analysis were within the normal range, which is considered to be less than 110 ms.44-46 In other words, the Pmax duration in PCOS patients is tuned in normal upper limits compared to non-PCOS. It can be assumed that being chronically in IR and hyperandrogenism states can lead to higher stages of atrial remodeling over time, slowed electric conduction, and longer P-wave duration. Our studied PCOS population was young and, therefore, might be in the early stages of PCOS and probably were not sufficiently exposed to IR and hyperandrogenism states to manifest noticeable and pathological atrial remodeling changes.
P dispersion (Pd) is a non-invasive ECG marker presenting intra- and inter-atrial conduction heterogeneity.47 Increased atrial heterogenic electrical activity by inducing atrial reentry leads to the development of cardiac arrhythmias (mostly AF and atrial flutter).22 Moreover, P-wave dispersion (Pd) is associated with IR independently but not with other factors such as BMI, waist circumference, LA size, LV diastolic function, and blood pressure.48 This meta-analysis revealed that Pd was significantly higher in PCOS patients than in controls, suggesting an increased susceptibility to develop AF compared to women without PCOS. Increased Pd in PCOS patients compared to controls were reported in all included studies in this meta-analysis.22-24,26-28 Heart rate was also significantly higher in PCOS patients than in controls. In a systematic review and meta-analysis that Wang et al48 conducted, it was concluded that there might be evidences of cardiovascular autonomic dysfunction in women with PCOS with increased sympathetic tone and decreased parasympathetic activity; in IR states, the sympathetic nervous system is hyperactive, which results in a significant prolongation of P-wave duration and Pd and increased heart rate.
Atrial electromechanical delay can also be measured from the onset of the P wave on ECG to the commencement of atrial contraction determined by pulsed-wave tissue Doppler imaging (TDI).15,49,50 In this meta-analysis, lateral PA and septal PA durations were significantly higher in PCOS patients than in controls, showing a possible electromechanical delay in the atrial conduction system; hence, we can say that longer Pmax duration, presence of P dispersion, and inter and delayed intra-atrial conduction define sinus impulses to have inhomogeneous propagation. This is a well-known ECG characteristic of the atrium that is likely to develop AF.51,52
In addition, enlargement and increased pressure of the atrium are known to affect P-wave disturbances.53-55 A larger left atrium (LA) diameter suggests early adverse cardiac modeling. Previous studies revealed that women with PCOS have greater left atrial dimensions than healthy controls.23,28,56,57 Zehir et al24 reported that prolonged atrial conduction time significantly correlated with IR, chronic inflammation, Pd, Left ventricular (LV) diastolic function parameters, and greater LA diameter.
LV diastolic dysfunction is an early echocardiographic manifestation of cardiomyopathy in patients with cardiovascular risk factors.58 There is evidence that patients with PCOS have elevated cardiovascular risk compared with age-matched controls. Higher incidence of obesity, impaired glucose tolerance, diabetes mellitus, and metabolic syndrome in PCOS patients define this association.59
Previous studies recommended that endothelial and LV diastolic dysfunction develops in patients with PCOS due to IR and hyperandrogenism.60,61 It is established that IR is associated with left ventricular (LV) remodeling independent from obesity and type-2 diabetes; LV remodeling leads to LV diastolic dysfunction.62,63
Ventricular filling pressure reflects LV diastolic function, and LA function is a significant determinant of LV diastolic filling. LA passive emptying volume depends on increased LV end-diastolic pressure.64,65
Multiple studies have assessed LV function in PCOS with echocardiographic parameters.23,24,27,28,49,56,57,66-69 It was revealed that there were significant differences in LV diastolic echo parameters between PCOS patients and control groups. The majority of these studies reported increased isovolumetric relaxation time (IVRT), deceleration time of early phase of mitral valve flow (DT), and a lower ratio of peak early and late diastolic flow velocities (E.A ratio) in PCOS patients compared to control group.22-24,28,56,68-70. Tíras et al reported a reverse correlation between insulin levels and the E.A ratio, suggesting that PCOS patients are probably more susceptible to developing diastolic dysfunction.68
In a study by Orio et al,57 PCOS women showed an increased LV mass index (LVMI) independent of body weight and hypertension. Moreover, De Jong et al66 and Yildirim et al67 also reported higher LVIM mass index in PCOS patients compared to control groups, suggesting PCOS could be considered an aggravating factor leading to LV hypertrophy and, therefore, to the early cardiovascular diseases in PCOS.
It is possible to say that changes in LV diastolic function indexes (IVRT, E.A ratio, DT, and LVMI) could be responsible for greater LA diameter and longer Pmax, Pd, inter, and intra-atrial conduction times in PCOS.
Obesity and IR are frequently seen in women with PCOS.71 By the high incidence of obesity and IR in PCOS, our results showed that PCOS patients have significantly higher BMI, HOMA-IR, and serum insulin levels than controls. In the study conducted by Bayir et al, it was revealed that there was a significant correlation between atrial conduction parameters and BMI in patients with PCOS despite a relatively lower range of BMI values in their study.23
Ventricular electrical activity and PCOS
Ventricular arrhythmia is defined as conditions that can range a spectrum of different abnormal cardiac rhythms from single premature ventricular complexes to polymorphic ventricular tachycardia and ventricular fibrillation. They are one of the most common causes of sudden cardiac death.72,73 Identifying the risk factors that generate Ventricular arrhythmias is essential. Using QT parameters, particularly QT dispersion, is a non-invasive method to measure ventricular electrical inhomogeneity.74 QTc interval has been proven to be related to cardiac arrhythmia and sudden death,75 same as QT dispersion.76,77 Çağlı et al78 reported that QT dispersion shows the heterogeneity of ventricular refractoriness, and prolonged QT dispersion has been correlated with the risk of arrhythmic death in various disorders (cardiac and non-cardiac). Also, a higher QRS-T angle can predict an increased risk of sudden cardiac death and other cardiovascular diseases.79,80 A study has described abnormal QRS-T angle as a probable predictor of ventricular arrhythmia.81 Through this, Topaloğlu et al82 assessed the QRS-T angle in women with PCOS and discovered abnormal degrees of QRS-T angle only in a small percentage of PCOS patients; interestingly, this abnormal angle turned out to be strongly correlated with mean ovarian volume (MOV). They concluded that MOV is a good predictor of abnormal QRS-T angle and cardiovascular diseases, considering that combined effects of IR and other hormonal parameters can influence MOV.
Gonadal steroids are essential determinants of gender-related differences in ventricular repolarization.83-85 It has been proven that by the onset of puberty, marked by increased testosterone concentration, the QTc interval starts to shorten in males, while in females, it stays unchanged.86 Testosterone appeared responsible for this sex difference by reducing action potential duration as its underlying mechanism.87 Vrtovec et al34 and Meden Vrtovec et al33 reported an inverse association between QT and QTc intervals and serum testosterone levels in PCOS patients, which means that they had shorter QTc intervals compared to controls. Huang et al11 concluded that although there was no significant difference between QT indices of PCOS patients and non-PCOS women, PCOS patients with androgen excess had shorter QT intervals in comparison with PCOS patients without androgen excess. On the other hand, Orio et al and Alpaslan et al reported that there were no significant differences in QT intervals and QT dispersion between PCOS patients and controls.18,19
This meta-analysis revealed no significant differences in QT parameters between the two groups of PCOS and controls except for the mean QTc parameter, which was significantly lower in PCOS patients compared to controls. Based on the testosterone effect on shortening QTc, this meta-analysis’s lower QTc interval in PCOS patients was probably associated with excess testosterone levels, significantly higher in PCOS patients than controls.
Clinical data indicates that both endogenous and exogenous estrogen can cause prolongation in QT interval and QT dispersion. Estrogens impact the activity of cardiac calcium currents, which determines the cardiac electrical cycle and the length of the action potentials and, thus, the QT interval length.84,88 Experimental animal studies revealed that estrogen prolongs the action potential’s QT interval and repolarization phase by depressing the potassium current expression.88,89 Studies show that hormone replacement therapy with estrogens in postmenopausal women prolonged QT intervals.90-92 Gazi et al reported significantly elevated serum estrogen and testosterone levels in PCOS patients that may explicate the prolonged QT dispersion in PCOS patients of their study.31 Both experimental and clinical findings support that estrogen and testosterone influence QTc. Estradiol levels were not significantly different between PCOS patients and controls in this meta-analysis; however, only 4 included studies evaluated estradiol levels in participants.
Some studies showed that hyperinsulinemia could prolong the QTc interval.93,94 Also, IR, a state of compensatory hyperinsulinemia, can persist QTc lengthening even when obesity, diabetes, and autonomic neuropathy are absent.95,96 QTc max, QTc min, and QT dispersion were longer in patients with metabolic syndrome.97 IR is involved in subclinical LV remodeling and dysfunction independent of traditional metabolic risk factors and can explain some impacts of BMI on concentric LV remodeling.62 Besides, it has been shown that LV remodeling alone is associated with malignant ventricular arrhythmias.98 Gazi et al reported that in patients with PCOS, HOMA-IR and insulin were associated with QTc prolongation despite the patients being non-obese.31 In our meta-analysis, serum insulin levels and HOMA-IR were significantly higher in PCOS patients compared to controls despite the non-significant difference in QT dispersion and shorter QTc interval in PCOS patients. Out of eleven studies enrolled in the meta-analysis, only five and three articles assessed HOMA-IR and serum insulin levels, respectively. It is good to mention that IR is not present in all patients with PCOS. Incidence of IR in PCOS has been reported up to 64% according to the HOMA-IR measurement.99
It is important to acknowledge certain limitations when comparing the provided reports. Firstly, the existing body of evidence on the relationship between cardiac electrical activity and PCOS in is still limited, with a relatively small number of studies available, most of which have been conducted before 2020.As a result, the certainty of evidence for the outcomes reported in this review is not yet high, and more original studies are needed to strengthen the conclusions.The limited number of studies included in the meta-analysis not only reduced the statistical power but also hindered the reliability of Egger’s and Begg’s tests and funnel plots for assessing publication bias.
Conclusion
This systematic review and meta-analysis demonstrate that PCOS is associated with impaired atrial electrical activity, evidenced by prolonged P-wave duration, increased P-wave dispersion, and delayed atrial electromechanical conduction. These findings suggest a heightened susceptibility to atrial arrhythmias, potentially driven by IR, chronic inflammation, and hyperandrogenism. In contrast, ventricular electrical parameters, including QT intervals and dispersion, showed no significant abnormalities, indicating no elevated risk of ventricular arrhythmias in PCOS. While the GRADE assessment highlighted variability in evidence certainty, these results underscore the need for further longitudinal studies to clarify long-term cardiovascular risks and mechanistic pathways in women with PCOS.
Competing Interests
The authors have no conflicts of interest to declare.
Ethical Approval
The protocol for this work was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (identifier: CRD42022337050).
Supplementary Files
Supplementary file 1 contains Table S1 (Search strategy performed in online databases) and Table S2 (Exclusion of studies based on inclusion and exclusion criteria during full text assessment).
(pdf)
Acknowledgements
We want to acknowledge all the healthcare providers of the Cardiology Department of Tabriz University of Medical Sciences for all the guides during the study.
References
-
Azziz R. Androgen Excess Disorders in Women. Springer; 2007. doi: 10.1007/978-1-59745-179-6.
- Livadas S, Diamanti-Kandarakis E. Polycystic ovary syndrome: definitions, phenotypes and diagnostic approach. Front Horm Res 2013; 40:1-21. doi: 10.1159/000341673 [Crossref] [ Google Scholar]
- Neven AC, Laven J, Teede HJ, Boyle JA. A summary on polycystic ovary syndrome: diagnostic criteria, prevalence, clinical manifestations, and management according to the latest international guidelines. Semin Reprod Med 2018; 36(1):5-12. doi: 10.1055/s-0038-1668085 [Crossref] [ Google Scholar]
- Ganie MA, Vasudevan V, Wani IA, Baba MS, Arif T, Rashid A. Epidemiology, pathogenesis, genetics & management of polycystic ovary syndrome in India. Indian J Med Res 2019; 150(4):333-44. doi: 10.4103/ijmr.IJMR_1937_17 [Crossref] [ Google Scholar]
- Melo AS, Vieira CS, Romano LG, Ferriani RA, Navarro PA. The frequency of metabolic syndrome is higher among PCOS Brazilian women with menstrual irregularity plus hyperandrogenism. Reprod Sci 2011; 18(12):1230-6. doi: 10.1177/1933719111414205 [Crossref] [ Google Scholar]
- Techatraisak K, Wongmeerit K, Dangrat C, Wongwananuruk T, Indhavivadhana S. Measures of body adiposity and visceral adiposity index as predictors of metabolic syndrome among Thai women with PCOS. Gynecol Endocrinol 2016; 32(4):276-80. doi: 10.3109/09513590.2015.1112785 [Crossref] [ Google Scholar]
- Vassilatou E, Lafoyianni S, Vryonidou A, Ioannidis D, Kosma L, Katsoulis K. Increased androgen bioavailability is associated with non-alcoholic fatty liver disease in women with polycystic ovary syndrome. Hum Reprod 2010; 25(1):212-20. doi: 10.1093/humrep/dep380 [Crossref] [ Google Scholar]
- Zhang J, Fan P, Liu H, Bai H, Wang Y, Zhang F. Apolipoprotein A-I and B levels, dyslipidemia and metabolic syndrome in south-west Chinese women with PCOS. Hum Reprod 2012; 27(8):2484-93. doi: 10.1093/humrep/des191 [Crossref] [ Google Scholar]
- Olufadi R, Byrne CD. Clinical and laboratory diagnosis of the metabolic syndrome. J Clin Pathol 2008; 61(6):697-706. doi: 10.1136/jcp.2007.048363 [Crossref] [ Google Scholar]
- Robinson S, Kiddy D, Gelding SV, Willis D, Niththyananthan R, Bush A. The relationship of insulin insensitivity to menstrual pattern in women with hyperandrogenism and polycystic ovaries. Clin Endocrinol (Oxf) 1993; 39(3):351-5. doi: 10.1111/j.1365-2265.1993.tb02376.x [Crossref] [ Google Scholar]
- Huang JH, Tsai JC, Hsu MI, Chen YJ. Cardiac conductive disturbance in patients with polycystic ovary syndrome. Gynecol Endocrinol 2010; 26(12):883-8. doi: 10.3109/09513590.2010.487593 [Crossref] [ Google Scholar]
- Faramawi MF, Delhey L, Abouelenein S, Delongchamp R. Metabolic syndrome and P-wave duration in the American population. Ann Epidemiol 2020; 46:5-11. doi: 10.1016/j.annepidem.2020.04.002 [Crossref] [ Google Scholar]
- Brand FN, Abbott RD, Kannel WB, Wolf PA. Characteristics and prognosis of lone atrial fibrillation 30-year follow-up in the Framingham Study. JAMA 1985; 254(24):3449-53. [ Google Scholar]
- Korantzopoulos P, Kolettis TM, Galaris D, Goudevenos JA. The role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillation. Int J Cardiol 2007; 115(2):135-43. doi: 10.1016/j.ijcard.2006.04.026 [Crossref] [ Google Scholar]
- Daubert JC, Pavin D, Jauvert G, Mabo P. Intra- and interatrial conduction delay: implications for cardiac pacing. Pacing Clin Electrophysiol 2004; 27(4):507-25. doi: 10.1111/j.1540-8159.2004.00473.x [Crossref] [ Google Scholar]
- Chan YH, Chang GJ, Lai YJ, Chen WJ, Chang SH, Hung LM. Atrial fibrillation and its arrhythmogenesis associated with insulin resistance. Cardiovasc Diabetol 2019; 18(1):125. doi: 10.1186/s12933-019-0928-8 [Crossref] [ Google Scholar]
- Fülöp L, Bányász T, Szabó G, Tóth IB, Bíró T, Lôrincz I. Effects of sex hormones on ECG parameters and expression of cardiac ion channels in dogs. Acta Physiol (Oxf) 2006; 188(3-4):163-71. doi: 10.1111/j.1748-1716.2006.01618.x [Crossref] [ Google Scholar]
- Orio F, Palomba S, Cascella T, Manguso F, Vuolo L, Tafuri D. Lack of electrocardiographic changes in women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2007; 67(1):46-50. doi: 10.1111/j.1365-2265.2007.02833.x [Crossref] [ Google Scholar]
- Alpaslan M, Onrat E, Yilmazer M, Fenkci V. QT dispersion in patients with polycystic ovary syndrome. Jpn Heart J 2002; 43(5):487-93. doi: 10.1536/jhj.43.487 [Crossref] [ Google Scholar]
- Balamurugan M, Maruthamuthu B, Ramanathan G. QT and corrected QT parameters in nonobese young Indian women with polycystic ovary syndrome. Int J Med Sci Public Health 2016; 5(12):2493-7. doi: 10.5455/ijmsph.2016.01052016502 [Crossref] [ Google Scholar]
- Aslan MM, Atıcı A, Cevrioğlu AS. Assessment of cardiac electrical activity in lean and obese patients with polycystic ovary syndrome. Cerrahpaşa Med J 2019; 43(2):40-3. doi: 10.5152/cjm.2019.19006 [Crossref] [ Google Scholar]
- Erdogan E, Akkaya M, Turfan M, Batmaz G, Bacaksız A, Tasal A. Polycystic ovary syndrome is associated with P-wave prolongation and increased P-wave dispersion. Gynecol Endocrinol 2013; 29(9):830-3. doi: 10.3109/09513590.2013.813474 [Crossref] [ Google Scholar]
- Türker Bayır P, Güray Ü, Duyuler S, Demirkan B, Kayaalp O, Kanat S. Assessment of atrial electromechanical interval and P-wave dispersion in patients with polycystic ovary syndrome. Anatol J Cardiol 2016; 16(2):100-5. doi: 10.5152/akd.2015.5735 [Crossref] [ Google Scholar]
- Zehir R, Karabay CY, Kocabay G, Kalayci A, Kaymaz O, Aykan AC. Assessment of atrial conduction time in patients with polycystic ovary syndrome. J Interv Card Electrophysiol 2014; 41(2):137-43. doi: 10.1007/s10840-014-9925-8 [Crossref] [ Google Scholar]
- Brennan SE, Munn Z. PRISMA 2020: a reporting guideline for the next generation of systematic reviews. JBI Evid Synth 2021; 19(5):906-8. doi: 10.11124/jbies-21-00112 [Crossref] [ Google Scholar]
- Akdag S, Cim N, Yildizhan R, Akyol A, Ozturk F, Babat N. Two markers in predicting the cardiovascular events in patients with polycystic ovary syndrome: increased P-wave and QT dispersion. Eur Rev Med Pharmacol Sci 2015; 19(18):3508-14. [ Google Scholar]
- Gazi E, Gencer M, Hanci V, Temiz A, Altun B, Barutcu A. Atrial conduction time, and left atrial mechanical and electromechanical functions in patients with polycystic ovary syndrome: interatrial conduction delay. Cardiovasc J Afr 2015; 26(6):217-21. doi: 10.5830/cvja-2015-046 [Crossref] [ Google Scholar]
- Taşolar H, Mete T, Ballı M, Altun B, Çetin M, Yüce T. Assessment of atrial electromechanical delay in patients with polycystic ovary syndrome in both lean and obese subjects. J Obstet Gynaecol Res 2014; 40(4):1059-66. doi: 10.1111/jog.12308 [Crossref] [ Google Scholar]
- Çimen T, Çakır E, Doğan M, Gökhan Vural M, Arslantaş U, Açıkel S. İdyopatik hirşutizm hastalarında QT dispersiyonu. Genel Tıp Dergisi 2013; 23(1):10-4. [ Google Scholar]
- Gateva A, Kamenov Z. Cardiovascular risk factors in Bulgarian patients with polycystic ovary syndrome and/or obesity. Obstet Gynecol Int 2012; 2012(1):306347. doi: 10.1155/2012/306347 [Crossref] [ Google Scholar]
- Gazi E, Gencer M, Hancı V, Temiz A, Altun B, Cakır Güngör AN. Relationship of QT dispersion with sex hormones and insulin in young women with polycystic ovary syndrome: an observational study. Anadolu Kardiyol Derg 2013; 13(8):772-7. doi: 10.5152/akd.2013.264 [Crossref] [ Google Scholar]
- Karaağaç K, Yontar OC, Emül A, Tenekecioğlu E, Erdolu M, Vatansever F. Tp-Te interval and Tp-Te/QT ratio in polycystic ovary syndrome. J Clin Anal Med 2015; 6(Suppl 1):5-8. doi: 10.4328/jcam.2578 [Crossref] [ Google Scholar]
- Meden-Vrtovec H, Vrtovec B, Osredkar J. Metabolic and cardiovascular changes in women with polycystic ovary syndrome. Int J Gynaecol Obstet 2007; 99(2):87-90. doi: 10.1016/j.ijgo.2007.06.005 [Crossref] [ Google Scholar]
- Vrtovec B, Meden-Vrtovec H, Jensterle M, Radovancevic B. Testosterone-related shortening of QTc interval in women with polycystic ovary syndrome. J Endocrinol Invest 2008; 31(7):653-5. doi: 10.1007/bf03345619 [Crossref] [ Google Scholar]
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J. GRADE guidelines: 1 Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011; 64(4):383-94. doi: 10.1016/j.jclinepi.2010.04.026 [Crossref] [ Google Scholar]
- Spach MS. Mounting evidence that fibrosis generates a major mechanism for atrial fibrillation. Circ Res 2007; 101(8):743-5. doi: 10.1161/circresaha.107.163956 [Crossref] [ Google Scholar]
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146(12):857-67. doi: 10.7326/0003-4819-146-12-200706190-00007 [Crossref] [ Google Scholar]
- Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998; 98(10):946-52. doi: 10.1161/01.cir.98.10.946 [Crossref] [ Google Scholar]
- Nichols GA, Reinier K, Chugh SS. Independent contribution of diabetes to increased prevalence and incidence of atrial fibrillation. Diabetes Care 2009; 32(10):1851-6. doi: 10.2337/dc09-0939 [Crossref] [ Google Scholar]
- Oliver-Williams C, Vassard D, Pinborg A, Schmidt L. Polycystic ovary syndrome as a novel risk factor for atrial fibrillation: results from a national Danish registry cohort study. Eur J Prev Cardiol 2021; 28(12):e20-2. doi: 10.1177/2047487320922927 [Crossref] [ Google Scholar]
- Zhou X, Dudley SC Jr. Evidence for inflammation as a driver of atrial fibrillation. Front Cardiovasc Med 2020; 7:62. doi: 10.3389/fcvm.2020.00062 [Crossref] [ Google Scholar]
- Zeller T, Schnabel RB, Appelbaum S, Ojeda F, Berisha F, Schulte-Steinberg B. Low testosterone levels are predictive for incident atrial fibrillation and ischaemic stroke in men, but protective in women - results from the FINRISK study. Eur J Prev Cardiol 2018; 25(11):1133-9. doi: 10.1177/2047487318778346 [Crossref] [ Google Scholar]
- Berger D, Folsom AR, Schreiner PJ, Chen LY, Michos ED, O’Neal WT. Plasma total testosterone and risk of incident atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Maturitas 2019; 125:5-10. doi: 10.1016/j.maturitas.2019.03.015 [Crossref] [ Google Scholar]
- Kitkungvan D, Spodick DH. Interatrial block: is it time for more attention?. J Electrocardiol 2009; 42(6):687-92. doi: 10.1016/j.jelectrocard.2009.07.016 [Crossref] [ Google Scholar]
- Ariyarajah V, Frisella ME, Spodick DH. Reevaluation of the criterion for interatrial block. Am J Cardiol 2006; 98(7):936-7. doi: 10.1016/j.amjcard.2006.04.036 [Crossref] [ Google Scholar]
- Bayés de Luna A, Platonov P, Cosio FG, Cygankiewicz I, Pastore C, Baranowski R. Interatrial blocks A separate entity from left atrial enlargement: a consensus report. J Electrocardiol 2012; 45(5):445-51. doi: 10.1016/j.jelectrocard.2012.06.029 [Crossref] [ Google Scholar]
- Centurión OA. Clinical implications of the P-wave duration and dispersion: relationship between atrial conduction defects and abnormally prolonged and fractionated atrial endocardial electrograms. Int J Cardiol 2009; 134(1):6-8. doi: 10.1016/j.ijcard.2008.12.072 [Crossref] [ Google Scholar]
- Wang W, Zhang F, Xhen J, Chen X, Fu F, Tang M. P-wave dispersion and maximum duration are independently associated with insulin resistance in metabolic syndrome. Ann Endocrinol (Paris) 2014; 75(3):156-61. doi: 10.1016/j.ando.2014.05.004 [Crossref] [ Google Scholar]
- Cui QQ, Zhang W, Wang H, Sun X, Wang R, Yang HY. Assessment of atrial electromechanical coupling and influential factors in nonrheumatic paroxysmal atrial fibrillation. Clin Cardiol 2008; 31(2):74-8. doi: 10.1002/clc.20162 [Crossref] [ Google Scholar]
- Pekdemir H, Cansel M, Yağmur J, Acikgoz N, Ermis N, Kurtoglu E. Assessment of atrial conduction time by tissue Doppler echocardiography and P-wave dispersion in patients with mitral annulus calcification. J Electrocardiol 2010; 43(4):339-43. doi: 10.1016/j.jelectrocard.2010.02.013 [Crossref] [ Google Scholar]
- Dilaveris PE, Gialafos EJ, Sideris SK, Theopistou AM, Andrikopoulos GK, Kyriakidis M. Simple electrocardiographic markers for the prediction of paroxysmal idiopathic atrial fibrillation. Am Heart J 1998; 135(5 Pt 1):733-8. doi: 10.1016/s0002-8703(98)70030-4 [Crossref] [ Google Scholar]
- Gialafos JE, Dilaveris PE, Gialafos EJ, Andrikopoulos GK, Richter DJ, Triposkiadis F. P-wave dispersion: a valuable electrocardiographic marker for the prediction of paroxysmal lone atrial fibrillation. Ann Noninvasive Electrocardiol 1999; 4(1):39-45. doi: 10.1111/j.1542-474X.1999.tb00363.x [Crossref] [ Google Scholar]
- Surawicz B. Electrocardiographic diagnosis of chamber enlargement. J Am Coll Cardiol 1986; 8(3):711-24. doi: 10.1016/s0735-1097(86)80207-8 [Crossref] [ Google Scholar]
- Chandraratna PA, Hodges M. Electrocardiographic evidence of left atrial hypertension in acute myocardial infarction. Circulation 1973; 47(3):493-8. doi: 10.1161/01.cir.47.3.493 [Crossref] [ Google Scholar]
- Josephson ME, Kastor JA, Morganroth J. Electrocardiographic left atrial enlargement Electrophysiologic, echocardiographic and hemodynamic correlates. Am J Cardiol 1977; 39(7):967-71. doi: 10.1016/s0002-9149(77)80209-9 [Crossref] [ Google Scholar]
- Tekin A, Tekin G, Cölkesen Y, Kiliçdağ EB, Başhan I, Sezgin AT. Left ventricular function in patients with polycystic ovary syndrome: a Doppler echocardiographic study. Exp Clin Endocrinol Diabetes 2009; 117(4):165-9. doi: 10.1055/s-2008-1080923 [Crossref] [ Google Scholar]
- Orio F Jr, Palomba S, Spinelli L, Cascella T, Tauchmanovà L, Zullo F. The cardiovascular risk of young women with polycystic ovary syndrome: an observational, analytical, prospective case-control study. J Clin Endocrinol Metab 2004; 89(8):3696-701. doi: 10.1210/jc.2003-032049 [Crossref] [ Google Scholar]
- Lin SL, Tak T, Kawanishi DT, McKay CR, Rahimtoola SH, Chandraratna PA. Comparison of Doppler echocardiographic and hemodynamic indexes of left ventricular diastolic properties in coronary artery disease. Am J Cardiol 1988; 62(13):882-6. doi: 10.1016/0002-9149(88)90886-7 [Crossref] [ Google Scholar]
- Apridonidze T, Essah PA, Iuorno MJ, Nestler JE. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2005; 90(4):1929-35. doi: 10.1210/jc.2004-1045 [Crossref] [ Google Scholar]
- Paradisi G, Steinberg HO, Hempfling A, Cronin J, Hook G, Shepard MK. Polycystic ovary syndrome is associated with endothelial dysfunction. Circulation 2001; 103(10):1410-5. doi: 10.1161/01.cir.103.10.1410 [Crossref] [ Google Scholar]
- Kelly CJ, Speirs A, Gould GW, Petrie JR, Lyall H, Connell JM. Altered vascular function in young women with polycystic ovary syndrome. J Clin Endocrinol Metab 2002; 87(2):742-6. doi: 10.1210/jcem.87.2.8199 [Crossref] [ Google Scholar]
- Shah RV, Abbasi SA, Heydari B, Rickers C, Jacobs DR Jr, Wang L. Insulin resistance, subclinical left ventricular remodeling, and the obesity paradox: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2013; 61(16):1698-706. doi: 10.1016/j.jacc.2013.01.053 [Crossref] [ Google Scholar]
- Yang CD, Shen Y, Lu L, Ding FH, Yang ZK, Zhang RY. Insulin resistance and dysglycemia are associated with left ventricular remodeling after myocardial infarction in non-diabetic patients. Cardiovasc Diabetol 2019; 18(1):100. doi: 10.1186/s12933-019-0904-3 [Crossref] [ Google Scholar]
- Rossi A, Zardini P, Marino P. Modulation of left atrial function by ventricular filling impairment. Heart Fail Rev 2000; 5(4):325-31. doi: 10.1023/a:1026507128973 [Crossref] [ Google Scholar]
- Aydin M, Ozeren A, Bilge M, Dursun A, Cam F, Elbey MA. Effects of dipper and non-dipper status of essential hypertension on left atrial mechanical functions. Int J Cardiol 2004; 96(3):419-24. doi: 10.1016/j.ijcard.2003.08.017 [Crossref] [ Google Scholar]
- De Jong KA, Berisha F, Naderpoor N, Appelbe A, Kotowicz MA, Cukier K. Polycystic ovarian syndrome increases prevalence of concentric hypertrophy in normotensive obese women. PLoS One 2022; 17(2):e0263312. doi: 10.1371/journal.pone.0263312 [Crossref] [ Google Scholar]
- Yildirim E, Karabulut O, Yuksel UC, Celik M, Bugan B, Gokoglan Y. Echocardiographic evaluation of diastolic functions in patients with polycystic ovary syndrome: A comperative study of diastolic functions in sub-phenotypes of polycystic ovary syndrome. Cardiol J 2017; 24(4):364-73. doi: 10.5603/CJ.a2017.0032 [Crossref] [ Google Scholar]
- Tíras MB, Yalcìn R, Noyan V, Maral I, Yìldìrìm M, Dörtlemez O. Alterations in cardiac flow parameters in patients with polycystic ovarian syndrome. Hum Reprod 1999; 14(8):1949-52. doi: 10.1093/humrep/14.8.1949 [Crossref] [ Google Scholar]
- Yarali H, Yildirir A, Aybar F, Kabakçi G, Bükülmez O, Akgül E. Diastolic dysfunction and increased serum homocysteine concentrations may contribute to increased cardiovascular risk in patients with polycystic ovary syndrome. Fertil Steril 2001; 76(3):511-6. doi: 10.1016/s0015-0282(01)01937-9 [Crossref] [ Google Scholar]
- Demirelli S, Degirmenci H, Ermis E, Inci S, Nar G, Ayhan ME. The importance of speckle tracking echocardiography in the early detection of left ventricular dysfunction in patients with polycystic ovary syndrome. Bosn J Basic Med Sci 2015; 15(4):44-9. doi: 10.17305/bjbms.2015.552 [Crossref] [ Google Scholar]
- Talbott E, Clerici A, Berga SL, Kuller L, Guzick D, Detre K. Adverse lipid and coronary heart disease risk profiles in young women with polycystic ovary syndrome: results of a case-control study. J Clin Epidemiol 1998; 51(5):415-22. doi: 10.1016/s0895-4356(98)00010-9 [Crossref] [ Google Scholar]
- Kuriachan VP, Sumner GL, Mitchell LB. Sudden cardiac death. Curr Probl Cardiol 2015; 40(4):133-200. doi: 10.1016/j.cpcardiol.2015.01.002 [Crossref] [ Google Scholar]
- Roberts-Thomson KC, Lau DH, Sanders P. The diagnosis and management of ventricular arrhythmias. Nat Rev Cardiol 2011; 8(6):311-21. doi: 10.1038/nrcardio.2011.15 [Crossref] [ Google Scholar]
- Higham PD, Campbell RW. QT dispersion. Br Heart J 1994; 71(6):508-10. doi: 10.1136/hrt.71.6.508 [Crossref] [ Google Scholar]
- Schouten EG, Dekker JM, Meppelink P, Kok FJ, Vandenbroucke JP, Pool J. QT interval prolongation predicts cardiovascular mortality in an apparently healthy population. Circulation 1991; 84(4):1516-23. doi: 10.1161/01.cir.84.4.1516 [Crossref] [ Google Scholar]
- Hii JT, Wyse DG, Gillis AM, Duff HJ, Solylo MA, Mitchell LB. Precordial QT interval dispersion as a marker of torsade de pointes Disparate effects of class IA antiarrhythmic drugs and amiodarone. Circulation 1992; 86(5):1376-82. doi: 10.1161/01.cir.86.5.1376 [Crossref] [ Google Scholar]
- Kuo CS, Reddy CP, Munakata K, Surawicz B. Mechanism of ventricular arrhythmias caused by increased dispersion of repolarization. Eur Heart J 1985; 6 Suppl D:63-70. doi: 10.1093/eurheartj/6.suppl_d.63 [Crossref] [ Google Scholar]
- Çağlı K, Ergün K, Lafçı G, Gedik HS, Ulaş MM. QT and P-wave dispersion. J Ankara Univ Fac Med 2005; 58(1):42-6. [ Google Scholar]
- Whang W, Shimbo D, Levitan EB, Newman JD, Rautaharju PM, Davidson KW. Relations between QRS-T angle, cardiac risk factors, and mortality in the third National Health and Nutrition Examination Survey (NHANES III). Am J Cardiol 2012; 109(7):981-7. doi: 10.1016/j.amjcard.2011.11.027 [Crossref] [ Google Scholar]
- Aro AL, Huikuri HV, Tikkanen JT, Junttila MJ, Rissanen HA, Reunanen A. QRS-T angle as a predictor of sudden cardiac death in a middle-aged general population. Europace 2012; 14(6):872-6. doi: 10.1093/europace/eur393 [Crossref] [ Google Scholar]
- Borleffs CJ, Scherptong RW, Man SC, van Welsenes GH, Bax JJ, van Erven L. Predicting ventricular arrhythmias in patients with ischemic heart disease: clinical application of the ECG-derived QRS-T angle. Circ Arrhythm Electrophysiol 2009; 2(5):548-54. doi: 10.1161/circep.109.859108 [Crossref] [ Google Scholar]
- Topaloğlu Ö, Çimci M, Yoloğlu S, Şahin İ. Is there association between QRS-T angle, and hormonal and sonographic features in polycystic ovarian syndrome?. Eur Rev Med Pharmacol Sci 2020; 24(13):7372-80. doi: 10.26355/eurrev_202007_21905 [Crossref] [ Google Scholar]
- Moss AJ. Measurement of the QT interval and the risk associated with QTc interval prolongation: a review. Am J Cardiol 1993; 72(6):23b-5b. doi: 10.1016/0002-9149(93)90036-c [Crossref] [ Google Scholar]
- Pham TV, Rosen MR. Sex, hormones, and repolarization. Cardiovasc Res 2002; 53(3):740-51. doi: 10.1016/s0008-6363(01)00429-1 [Crossref] [ Google Scholar]
- Bidoggia H, Maciel JP, Capalozza N, Mosca S, Blaksley EJ, Valverde E. Sex differences on the electrocardiographic pattern of cardiac repolarization: possible role of testosterone. Am Heart J 2000; 140(4):678-83. doi: 10.1067/mhj.2000.109918 [Crossref] [ Google Scholar]
- Rautaharju PM, Zhou SH, Wong S, Calhoun HP, Berenson GS, Prineas R. Sex differences in the evolution of the electrocardiographic QT interval with age. Can J Cardiol 1992; 8(7):690-5. [ Google Scholar]
- Sedlak T, Shufelt C, Iribarren C, Merz CN. Sex hormones and the QT interval: a review. J Womens Health (Larchmt) 2012; 21(9):933-41. doi: 10.1089/jwh.2011.3444 [Crossref] [ Google Scholar]
- Drici MD, Burklow TR, Haridasse V, Glazer RI, Woosley RL. Sex hormones prolong the QT interval and downregulate potassium channel expression in the rabbit heart. Circulation 1996; 94(6):1471-4. doi: 10.1161/01.cir.94.6.1471 [Crossref] [ Google Scholar]
- Ebert SN, Liu XK, Woosley RL. Female gender as a risk factor for drug-induced cardiac arrhythmias: evaluation of clinical and experimental evidence. J Womens Health 1998; 7(5):547-57. doi: 10.1089/jwh.1998.7.547 [Crossref] [ Google Scholar]
- Haseroth K, Seyffart K, Wehling M, Christ M. Effects of progestin-estrogen replacement therapy on QT dispersion in postmenopausal women. Int J Cardiol 2000; 75(2-3):161-5; discussion 5. doi: 10.1016/s0167-5273(00)00317-x [Crossref] [ Google Scholar]
- Carnethon MR, Anthony MS, Cascio WE, Folsom AR, Rautaharju PM, Liao D. A prospective evaluation of the risk of QT prolongation with hormone replacement therapy: the atherosclerosis risk in communities study. Ann Epidemiol 2003; 13(7):530-6. doi: 10.1016/s1047-2797(03)00050-4 [Crossref] [ Google Scholar]
- Yildirir A, Aybar F, Kabakci MG, Yarali H, Akgul E, Bukulmez O. Hormone replacement therapy shortens QT dispersion in healthy postmenopausal women. Ann Noninvasive Electrocardiol 2001; 6(3):193-7. doi: 10.1111/j.1542-474x.2001.tb00107.x [Crossref] [ Google Scholar]
- Gastaldelli A, Emdin M, Conforti F, Camastra S, Ferrannini E. Insulin prolongs the QTc interval in humans. Am J Physiol Regul Integr Comp Physiol 2000; 279(6):R2022-5. doi: 10.1152/ajpregu.2000.279.6.R2022 [Crossref] [ Google Scholar]
- Van De Borne P, Hausberg M, Hoffman RP, Mark AL, Anderson EA. Hyperinsulinemia produces cardiac vagal withdrawal and nonuniform sympathetic activation in normal subjects. Am J Physiol 1999; 276(1):R178-83. doi: 10.1152/ajpregu.1999.276.1.R178 [Crossref] [ Google Scholar]
- Frank S, Colliver JA, Frank A. The electrocardiogram in obesity: statistical analysis of 1,029 patients. J Am Coll Cardiol 1986; 7(2):295-9. doi: 10.1016/s0735-1097(86)80494-6 [Crossref] [ Google Scholar]
- Kahn JK, Sisson JC, Vinik AI. QT interval prolongation and sudden cardiac death in diabetic autonomic neuropathy. J Clin Endocrinol Metab 1987; 64(4):751-4. doi: 10.1210/jcem-64-4-751 [Crossref] [ Google Scholar]
- Soydinc S, Davutoglu V, Akcay M. Uncomplicated metabolic syndrome is associated with prolonged electrocardiographic QTc interval and QTc dispersion. Ann Noninvasive Electrocardiol 2006; 11(4):313-7. doi: 10.1111/j.1542-474X.2006.00123.x [Crossref] [ Google Scholar]
- Azevedo PS, Polegato BF, Minicucci MF, Paiva SA, Zornoff LA. Cardiac remodeling: concepts, clinical impact, pathophysiological mechanisms and pharmacologic treatment. Arq Bras Cardiol 2016; 106(1):62-9. doi: 10.5935/abc.20160005 [Crossref] [ Google Scholar]
- DeUgarte CM, Bartolucci AA, Azziz R. Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertil Steril 2005; 83(5):1454-60. doi: 10.1016/j.fertnstert.2004.11.070 [Crossref] [ Google Scholar]