Biomed. adv. 2(1):10-24.
doi: 10.34172/bma.10
Review Article
Therapeutic effects of NK cell-derived EVs on cancer: Current advances and future treatment strategies
Mehtap Kizilpinar Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, 1 
Mohammadreza Dastouri Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – review & editing, 2, * 
Author information:
1Ankara University Biotechnology Institute and Sisbiyotek Advanced Research Unit, Gumusdere Yerleskesi, Kecioren, Ankara, Turkey
2Department of Medical Biology and Genetics, Faculty of Medicine, Ankara Medipol University, Eti, Celal Bayar, Cankaya /Ankara, Turkey
Abstract
Summary
Cancer remains a leading cause of death globally, with conventional treatments such as surgery, chemotherapy, and radiotherapy offering limited success, especially in advanced stages or cases with metastatic or resistant tumors. As a result, there is a pressing need for novel therapeutic approaches. Immunotherapy has emerged as a promising alternative, with natural killer (NK) cells playing a central role due to their ability to selectively target and kill cancer cells while sparing healthy tissues. This review explores the therapeutic potential of NK cell-derived extracellular vesicles (NK-EVs), a promising innovation in cancer treatment. NK-EVs, including exosomes, carry bioactive molecules such as proteins, lipids, and RNA that can influence tumor cells and the surrounding immune microenvironment. The review examines the various sources of NK-EVs, their biological functions, and the mechanisms through which they exert their therapeutic effects, focusing on both their membrane and content proteins. These vesicles play a crucial role in immune modulation, including enhancing immune responses and promoting apoptosis in cancer cells. Additionally, NK-EVs have gained attention as potential carriers for delivering therapeutic agents, such as chemotherapeutic drugs, small interfering RNAs (siRNAs), and microRNAs (miRNAs), providing a novel approach for overcoming the limitations of traditional cancer therapies, such as poor drug delivery and resistance. The review also highlights recent advances in NK-EV production, including bioreactor-based systems and stress-induced EV generation, which are essential for scaling up their use in clinical applications. Furthermore, we discuss the immunotherapeutic effects of NK-EVs and NK exosomes (Exos), the methods for assessing their cytotoxicity, and their potential to serve as effective carriers for targeted therapy. By reviewing current advancements and future strategies, this article provides a comprehensive outlook on NK-EVs as a promising tool in the fight against cancer, offering novel pathways for more effective and targeted cancer therapies.
Keywords: Cancer therapy, NK cells, NK-EVs, Exosome
Copyright and License Information
© 2025 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
None.
Introduction
Cancer and the treatment approaches
Cancer is one of the deadliest diseases affecting the whole world and occurs in a process that includes multi-step malignant transformation stages from neoplasia. The complex biological mechanism of cancer and the variety of its types and subtypes make it difficult to develop an effective treatment method. Radiation, chemotherapy, surgical resection, and combinations of these methods have been used for cancer treatment for many years. However, these treatments are often inadequate. For this reason, developing new treatment methods against cancer is essential. In the relevant section, standard treatment methods and new potential treatments developed against cancer to date are included.
Standard cancer therapies and their limitations
Traditional methods used in cancer treatment are surgical resection, chemotherapy, and/or radiotherapy. Surgical resection is a very effective method in early-stage cancer diagnosis, but unfortunately, it may be insufficient in metastatic stages. In addition, chemotherapy and radiotherapy can damage healthy cells as well as eliminate cancer cells. (Figure 1) Additionally, over time, drug resistance mechanisms are seen to develop against chemotherapy agents may be insufficient when applied alone.1,2 In addition, patient body resistance is also considered when applying chemotherapeutic agents in patients diagnosed at an advanced age, and treatment tolerance is an important factor.3
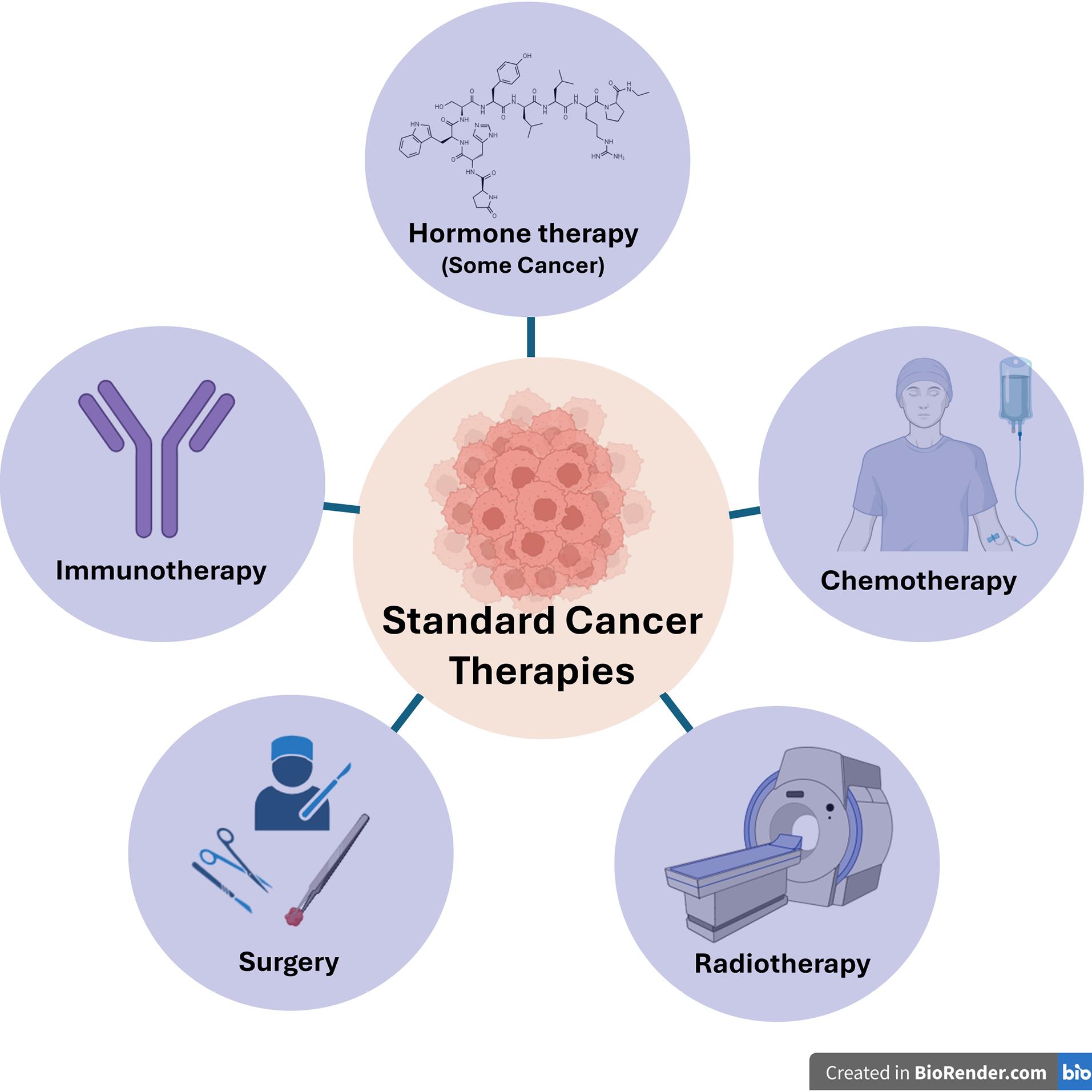
Figure 1.
Standard cancer therapies
.
Standard cancer therapies
Additional treatments are needed to increase the effectiveness of chemotherapy. Some studies have shown that restrictive factors such as mutations have a negative effect on the application of relevant drug treatments. For example, after enzalutamide treatment, point mutations in the androgen receptor (AR) can occur as AR-dependent, such as gene amplification and overexpression, or as AR-independent, such as epithelial-mesenchymal transition and up-regulation of the glucocorticoid receptor. These changes cause drug resistance on prostate cancer.4
Based on the relevant results, mutation types are important markers that determine the course and effectiveness of treatment.
In summary, in cancer treatment, there are various parameters, such as the patient’s age and general condition, the stage of the disease, and the tumor subtype, which determine the treatment method and its effect. As mentioned above, each treatment method is preferred depending on the relevant conditions and shows different side effects. It is crucial to develop targeted treatments to minimize treatment failure and increase effectiveness as much as possible.
Since its inception, immunotherapy has garnered much interest as it becomes the cornerstone of the new wave of cancer treatments. It is a treatment approach that sparingly causes adverse effects when compared with chemotherapy. It boosts the patient’s survival rate in several forms of cancer, justifying the need to explore more immunotherapy.5
Natural killer (NK) cell and immunotherapy
NK cells are lymphocyte groups that exhibit cytotoxic effects against abnormal cell groups and pathogen infections, including tumor cells, in the early stages of immune responses without the need for prior sensitization of target cells.6
While NK cells originate from the same early progenitor as T cells, they do not mature in the thymus gland as T cells do. Unlike T cells, NK cells lack antigen-specific receptors and CD3. The markers commonly used in clinical settings are CD16 (FcRγ) and CD56 (CAM).7
NK cells possess activating (KAR) and inhibitory (KIR) receptors that detect unusual changes in the extracellular environment.8 In the absence of pathogens and tumor cells, the inhibitory receptors on NK cell membranes engage with human leukocyte antigen (HLA), preventing the activation of specific molecules by the activating receptors.8 This mechanism helps maintain control over NK cell activity. Receptors on NK cells, such as NKp30, NKp44, NKp46, and DNAM-1, interact with target cells, exerting cytotoxic effects against abnormal cells during the initial phase of immune response.9
NK cells contain a cytoplasm that is abundant in granules. When an abnormal target cell interacts with an NK cell, a synapse triggers the release of lytic granules.10 These granules are related to lysosomes, and perforins create osmotic lysis in the target cell’s membrane by forming pores. Secreted granzymes from NK cells enter these pores and activate caspases.10 The direct killing effect can result from granules released by NK cells, Fas ligand, and TNF-related apoptosis-inducing ligand (TRAIL)11 (Figure 2).
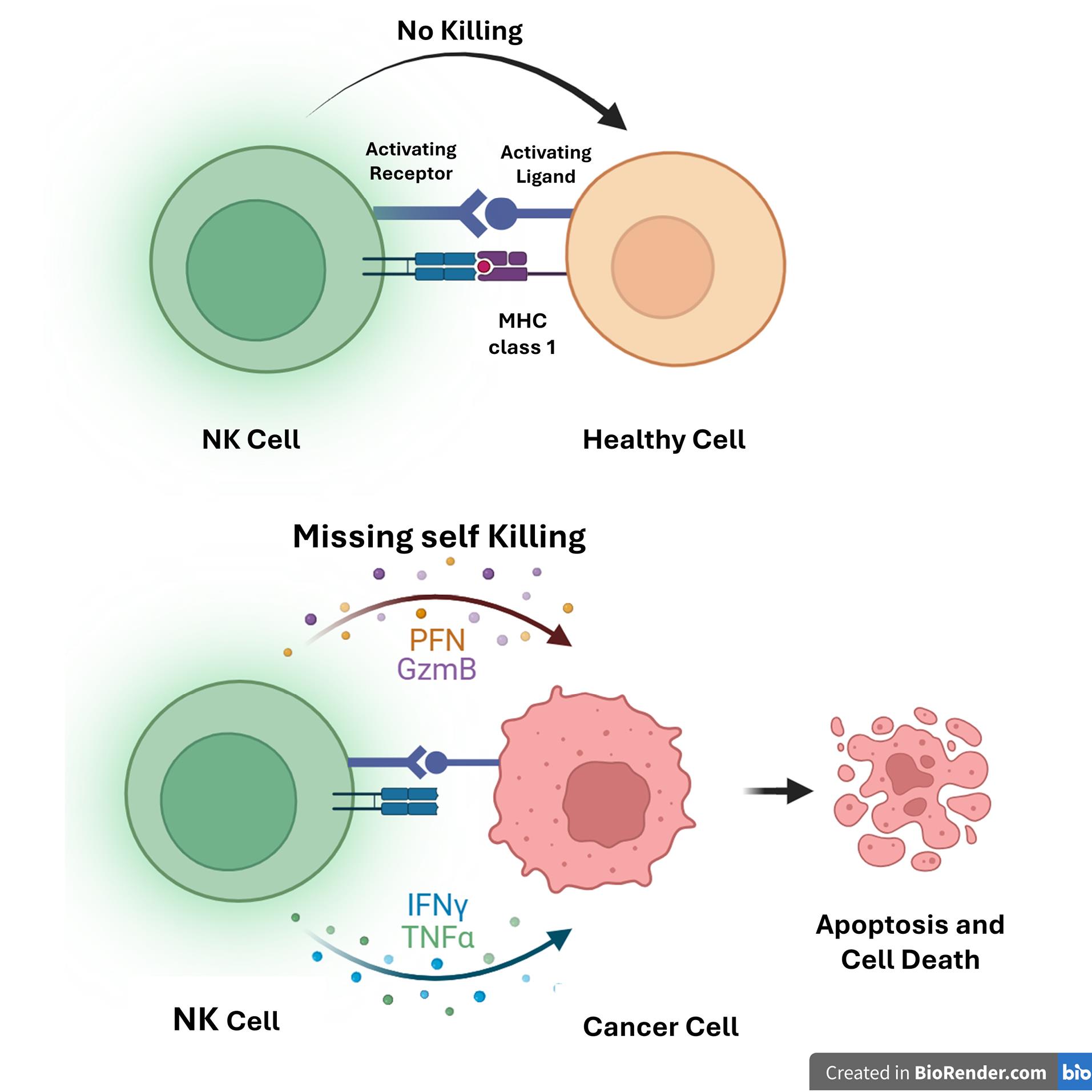
Figure 2.
Mechanisms of recognition and killing of defective cells by natural killer cells
.
Mechanisms of recognition and killing of defective cells by natural killer cells
Various studies have shown that the notable features of NK cells have led researchers to explore their potential in immunotherapy.12 In investigations focused on NK-based cellular therapies, allogeneic NK cells or modified NKs with chimeric antigen receptors (CARs) have been employed against different cancer types, with evaluations of their antitumor effects.13 These studies have yielded promising results, including tumor elimination that does not rely on receptors.14 Nonetheless, there are drawbacks, such as the limited ability to infiltrate solid tumors, challenges in producing enough ex vivo, a short in vivo half-life, and the necessity for regular supplementation. Thus, the direct application of these cells in immunotherapy is quite complex.15
NK cell-derived extracellular vesicles (EVs) and Immunotherapy
A. Extracellular Vesicles
Intercellular communication is crucial whether the microorganism is simple or complex. Research to understand this mechanism has shown that many eukaryotic organisms release membrane-derived soluble factors into their surrounding environment.16 Two studies from the 1980s observed the release of multivesicular bodies from reticulocyte cells into the extracellular space.17,18
Subsequent studies revealed that nearly all mammalian cells and various cancer cell lines release these relevant vesicles.19-21 Over the years, it became clear that these vesicles were not merely dysfunctional; they played a crucial role in intercellular communication by carrying factors like proteins and lipids and even helping stimulate immune responses.22,23
EVs are divided into three classes, according to their biogenesis: exosomes (30-150 nm), microvesicles (150-1000 nm), and apoptotic vesicles (50-2000 nm). Each subclass of EVs has specific phenotype, protein, and molecule ingredients.24 Exosomes are released to the microenvironment from the endolysosomal pathway. However, microvesicles and apoptotic vesicles are derived from the plasma membrane. Due to microvesicles derived from the plasma membrane directly, microvesicles are more like the membrane composition of the main cell compared to endosomal-derived exosomes. While exosomes and microvesicles can contain cytoplasmic and membrane proteins, including miRNAs and receptors, apoptotic bodies can consist of nuclear fraction and cell organelles.25
Different isolation methods are used to obtain EVs. Ultracentrifugation is the most used EV isolation method. In addition to this relevant method, various techniques such as density gradient centrifugation, differential centrifugation, size exclusion chromatography (SEC), and polyethylene glycol 8000 precipitation can increase purity.26 Ultracentrifugation isolation is an easy-to-use, high-efficiency, and noncomplex method. However, it is also time-consuming, and product recovery is variable and correlates with low purity. Although isolation techniques are various, achieving efficient recovery and high specificity is quite challenging. 27 So, there is a need to develop different techniques.
B. Natural killer cell-derived extracellular vesicles
EVs are significant in various physiological and pathological processes.28 Research indicates that NK-EVs, like NK cells, can directly eliminate tumor cells due to their cytotoxic proteins and cytokines content. Additionally, they can interact with immune cells within the tumor microenvironment, influencing the behavior of these cells.29
NK-EVs have cytotoxic proteins and cell surface receptors that function similarly to NK cells from which they originated, and they exhibit apoptotic effects against cancer cells.6 EVs derived from NK cells carry cytotoxic proteins in their structures, including granzymes, perforin, granulysin, TNF-related apoptosis-inducing ligands (TRAIL/CD253), FasL/CD178, and small antimicrobial peptides.30 NK-EVs may also indirectly induce abnormal cell death using immunomodulation by containing molecules with adhesion and homing properties.31 Given these characteristics, a comprehensive understanding and analysis of NK-EVs could be crucial for their potential as future treatment options.
C. Sources of NK-EVs
The sources from which NK-EVs are obtained may vary. NKs can be obtained from peripheral blood mononuclear cells (PBMCs) or cell lines.32 PBMCs can be collected from healthy or diseased living donors. Although NK cells obtained from PBMC collected from cancer patients have a safe profile based on their low adverse effects, the cytotoxic effect of EVs derived from these cells has been found to be low compared to other NK cell sources.33,34 In contrast, allogeneic NK cells from healthy donors were more cytotoxic and had a higher side effect profile.28 In addition, it is challenging to collect NK-derived EVs and apply them as a treatment because large amounts of product are difficult to obtain, and there are limited blood groups and donors.35
As an alternative to PBMCs, using cell lines to obtain NK-EVs is advantageous on many sides. NK-92 and NK-92MI cell lines are commonly used as sources. The NK-92 cell line is the only human NK cell line with FDA approval for clinical use. NK-92 cell line is easier to use and apply and less costly. Relevant cell lines can be maintained in culture by stimulating it with cytokines.36 In a study, EVs derived from primary NK cells and EVs derived from NK-92 cell lines showed similar profiles in terms of cytotoxic protein content.37
Recently, different alternatives have been offered as sources of NK-EVs. Relevant sources are umbilical cord blood, CAR NK, induced pluripotent stem cells and hematopoietic stem and progenitor cells, and these cell sources are open to development and manipulation38,39 (Figure 3). In the future, it is predicted that the sources of EVs will become more accessible to obtain.
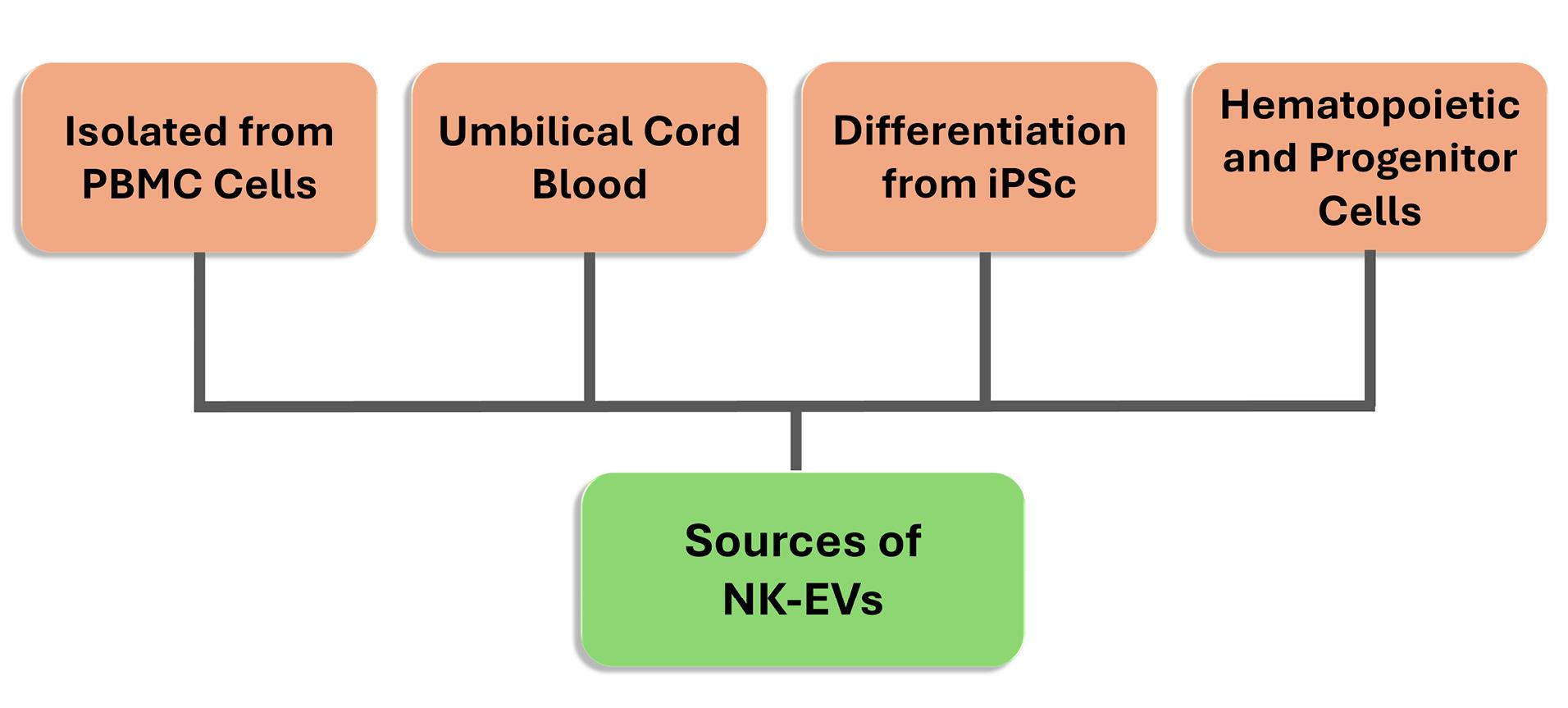
Figure 3.
The Sources of NK-EVs
.
The Sources of NK-EVs
In addition to the advantage of NK cells in cancer targeting, EVs can easily infiltrate the tumor microenvironment.40 In addition, features such as being biocompatible, acting on target, being safe regarding side effect profile, and having intense penetration characterize the strengths of these cells.6 Besides directly targeting and eliminating cancer cells with the surface receptors they carry, NK-EVs can also act as carrier cargo. NK-EVs can deliver various chemotherapeutic agents and miRNAs to cancer cells.41,42 Various studies are being carried out to increase NK-EVs’ functionality on the relevant properties, which are promising.43
D. Biological functions of natural killer cell extracellular vesicles
NK-EVs have cytotoxic and immunomodulatory effects like the NK cells from which they originate. While NK-EVs show their cytotoxic effects through the proteins they contain, they also show their immunomodulatory effects by affecting immune cells in the tumor microenvironment.44 In this section, we will discuss the biological functions of NK-EVs.
D.1. Functional mechanisms of membrane and content proteins of NK-EV
NK-EVs carry bioactive molecules such as cytotoxic proteins and miRNAs.45,46 Cytotoxic proteins can kill the target cell via caspase-dependent or caspase-independent pathways.46 the caspase-independent apoptosis pathway, granzyme A triggers the disassembly of the SET protein complex and causes single-stranded DNA damage in the target cell. Granzyme B activates procaspase in the caspase-dependent pathway, releasing cytochrome c from the mitochondria. Cytochrome c activates caspase-3, then caspases 7 and 9 are activated, and apoptosis is induced. Granulysin damages cell membrane integrity and induces ER stress apoptosis. Caspase signaling and mitochondrial and ER stress play essential roles in NK-EV-induced cytotoxicity. Perforin, a cytotoxic protein in NK-EVs, embeds into the target cell membrane, forming a pore. Thus, this pore provides an environment for granzymes to enter.46
FasL is a type II transmembrane protein part of the tumor necrosis factor superfamily comprising death factors. It can trigger the exogenous apoptotic pathway by activating poly(ADP-ribose) polymerase (PARP) with caspase-3 and caspase-8. They can be found in soluble and membrane-bound forms. The inhibitory effect of FasL in NK-EVs on cancer cells is still controversial. One study suggested that the impact of FasL in NK exosomes on cytotoxicity was relatively low, while another study demonstrated efficacy using an anti-Fas antibody.46,47 More studies are needed on this subject.
D.2.Immunomodulatory effect mechanism of NK-EVs
As mentioned above, EVs are secreted by almost all mammalian and immune cells.30 Studies have determined that EVs derived from immune cells play an immunomodulatory role in the tumor microenvironment.25,48 If we examine the relevant functions in terms of NK-EVs, it has been determined that exosomes reduce the number of tumor precursor M2 macrophages or increase tumor suppressor M1 macrophage polarization49 and induce indirect T cell proliferation by T cell activation or increased co-stimulatory expression in monocytes.28 In another study, miRNA analysis in NK-EVs revealed that they promote T cell activation and induce dendritic cell expression of MHC-II and CD86.50 NK-EVs have also been shown to stimulate NK cells, especially the CD56bright phenotype, which is the most effective type in terms of cytokine production.51,52 In addition, NK-EVs contain many molecules related to the immune response, including cytokines such as TNF-α, IFN-γ, and IL-6, but their effects must be investigated.53
The potential immunomodulatory effect of NK-EVs on cancer cells and their ability to eliminate tumor immunosuppression make them a unique candidate for cancer immunotherapy. Future studies will effectively use these properties.
Utilizing NK cell or activated NK cells to cancer treatment
Immunotherapy is an innovative treatment method that activates the body’s defense mechanism by triggering the immune system.54 In recent years, successful results have been obtained with immunotherapy in many types of cancer, while studies are still ongoing.55,56 Dastouri et al illustrated the potential of CD226-stimulated NK-92 (sNK-92) cells in breast cancer immunotherapy in 2023. In this study, MDA-MB-231 triple-negative breast cancer cells were selectively targeted by sNK-92 cells, demonstrating a stronger cytotoxic effect than regular NK-92 cells without affecting normal breast cells (MCF-12A). Increased granzyme B secretion and higher levels of apoptosis-related proteins (BAX, CASP3, and CASP9) were observed in cancer cells co-cultured with sNK-92 cells, indicating specific targeting.57
Current treatment methods focus on immune checkpoint inhibitors, CAR T cells, and bispecific T cell-engaging antibodies.58-60 Studies on immune checkpoint inhibitors are predominantly conducted on inhibiting cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) receptors.61,62 Antibodies targeting immune checkpoints, such as ipilimumab (anti-CTLA-4) and nivolumab (anti-PD-1), have been investigated in treating prostate cancer as monotherapy or combined therapy.63,64 As a result of clinical studies, it has been observed that low immune infiltration in some cancer types, which is a “cold tumor,” reduces the effectiveness of immunotherapy.65 To overcome this obstacle, various trials have been carried out in which chemotherapy-targeted therapy and receptor inhibitory agents were combined.66,67 Checkpoint inhibitors’ limited effectiveness is due to prostate cancer’s low tumor mutation load, low T cell infiltration, and cold tumor environment.68 In addition, factors such as TGF-Beta, VEGF, and IL10 secreted by cancer cells into the microenvironment increase resistance to the relevant treatment method.69,70
Bispecific antibodies targeting T cell costimulatory receptors show their effect by targeting a selected tumor antigen and a T cell costimulatory receptor. With this antibody-dependent targeting, cytotoxic activation of the T cell is activated by providing an immunological synapse between tumor cells and T cells.71 Bispecific antibody therapies have limitations such as short half-life, the need for frequent dosing, and the risk of excessive cytokine release in treated patients, necessitating close monitoring of patients.72 Therefore, we need to develop the relevant treatment method.
CAR-T therapy is a form of adoptive cell immunotherapy in which patient-derived T lymphocytes are genetically modified to express synthetic receptors directed against tumor-associated antigens.73 This treatment method recognizes and destroys cancer cells independently of MHC.73 However, CAR T therapies have potential serious side effects, such as susceptibility to infections, cytokine release syndrome, and immune effector cell-associated neurotoxicity syndrome.74,75 Consequently, further studies are needed to improve the therapeutic efficacy of cancer treatment.
Strategies for utilizing NK cell-derived EVs/Exos in cancer therapy
NK cells are among the first immune elements involved in the fight against cancer cells and respond very quickly.76 There are various studies in which NK cells are used directly modified with CARs for treatment purposes.77,78 According to the obtained data, the effect of these cells on solid tumors is low due to their inability to penetrate the tumor microenvironment.79 However, EVs derived from NK cells contain cytolytic proteins and are smaller in size, thus easier to access tumor cells, making EVs advantageous in showing an apoptotic effect against cancer cells.80 In addition, EVs have the advantage of showing the characteristics of the cell from which they originate and therefore EVs obtained from NKs have the potential to carry NK properties. Studies prove these structures have anti-proliferative effects on melanoma, colon, prostate, glioma, breast and ovarian cancer cells.80
Immunotherapeutic effect of NK-EVs
Considering the recent studies conducted to investigate the effect of NK-EVs on cancer cells, most of them are based on the stimulation of NK cell lines such as NK-92 by treating them with interleukin derivatives and analyzing the apoptotic effect. In a study, NK-EVs were isolated from a culture medium with or without adding IL-15, purified by density gradient ultracentrifugation (DG-UC), and then characterized. Their anti-tumor effect on various cancer cells was evaluated. As a result, IL-15-treated NK-EVs showed significantly higher cytolytic activity against human glioblastoma, breast cancer, and thyroid cancer cell lines. The expression of molecules associated with NK cell cytotoxicity was increased. IL-15-treated NK-EVs significantly inhibited the growth of xenograft glioblastoma cells in mice. Thus, it has been suggested that IL-15 may improve the immunotherapeutic effect of NK-EVs.81
In a recent study by Aarsund et al, NK-EVs obtained from primary NK cells, NK-92, and KHYG-1 NK cell lines were cultured with IL-15 alone or in combination with IL-12 and IL-18 to investigate their tumor-targeting potential. Tumor cell apoptosis was measured on human breast, colon, ovary, glioblastoma, and prostate cell lines. The efficiency of EVs obtained from stimulated cells was observed to be higher than that of EVs obtained from resting cells.80 Proteomic analysis showed that cytolytic proteins were similarly distributed in EVs obtained from primary NK cells and NK-92 cells. However, EVs obtained from KHYG-1 cells tended to exhibit lower capacity to target tumor cells. As a result, EVs derived from stimulated primary NK cells or NK-92 cells have been found to have the best potential to target solid tumors and infiltrate the microenvironment.80
In another study conducted in 2022, EVs obtained from NK92 cells were stimulated with various IL groups to investigate their cytotoxic capacity against cancer cells. The relevant study showed that the cytotoxic capacity of NK-EVs stimulated with the combination of IL-15 + IL-21 increased against cancer cells. It was shown that the significant cytolytic granules, granzyme B and granzyme H, were enriched in EVs with IL stimulation.82 In knockout experiments of cytolytic granules, it was determined that the relevant structures were independent of the increased cytotoxic capacity. Mass spectrometry experiments were performed to determine the key molecules playing a role in this mechanism. It has been found that the activating receptor CD226 (DNAM-1) on NK-EVs is enriched by IL stimulation, and cytotoxic activity decreases after blocking CD226 with antibodies.82
In an in vitro study conducted by Jong et al in 2017, the cytotoxic effect of EVs obtained from PB-NK of healthy donors was tested on human acute lymphoblastic leukemia (NALM-6; SUPB15), neuroblastoma (CHLA-136; CHLA-255), and breast cancer (MCF-7) cell lines. The relevant NK-EVs increased apoptosis in all tumor cell lines in a time- and dose-dependent manner.83
In another in vitro study, the potential of NK-EVs obtained from PB-NK of healthy donors and human NK cell line (NK92-MI) on human acute lymphoblastic leukemia (SUPB15) and neuroblastoma (CHLA255) cancer cell lines was investigated. It was determined that PFN, GzmA, GzmB, and GNLY work together to produce a potent cytotoxicity effect, offering hope for the future of cancer treatment. The anti-tumor effect is provided with different alternatives by caspase-dependent and caspase-independent pathways.46
Another study investigating the cytotoxic effect of EVs derived from NK3.3 cells on cancer cells aimed to obtain an environment close to in vivo using a three-dimensional MCF7 breast cancer mammosphere model. NK3.3-EVs penetrated MCF7 mammospheres and caused death through apoptosis. In addition, in another set of experiments targeting first-line chemotherapy-resistant K562 chronic myeloid leukemia tumor cells, NK-EVs were found to have an effect against the cancer stem cell (CSC) population by reducing the expression of CSC-related tumor-promoting genes.84
In a study to increase the efficacy of adoptive T cell therapy on solid tumors, EVs derived from NK92 cells were used for a synergistic result. The experiments supported the effect of CTL through multiplex pathways such as induction of tumor apoptosis, increased MHC-I expression in tumor cells, and reprogramming of tumor-associated macrophages from pro-tumoral M2 phenotypes to tumoricidal M1 phenotypes. The overall coordination of NK-EV with CTL resulted in strong tumor suppression.31
Although studies conducted to investigate NK-EVs’ potential as cancer therapeutic agents are predominantly in vitro, there are also some in vivo studies. One study investigated the antitumor effects of NK-92MI exosomes in hepatocellular carcinoma (HCC) using an orthotopic and subcutaneous tumor model. It was observed that NK exosomes expressed both typical exosomal markers (e.g., CD63, CD81, and Alix) and cytotoxic proteins (e.g., perforin, granzyme B, FasL, and TRAIL). While the NK exosome was selectively taken up by HCC cells (e.g., Hep3B, HepG2, and Huh 7), the highest cytotoxic effect appeared to be induced on Hep3B cells. NK-Exo has been shown to inhibit phosphorylation of serine/threonine protein kinases (e.g., AKT and ERK1/2) and increase activation of specific apoptosis markers (e.g., caspase-3, -7, -8, -9, and PARP) in Hep3B cells. NK exosomes have also exhibited potent therapeutic effects in orthotopic and subcutaneous HCC mouse models.85
In a recent study, NK EV was applied in vitro and in vivo on the Osimertinib-resistant H1975 lung cancer cell line containing the L858R EGFR mutation, alone or in combination with carboplatin. The results showed that NK-EVs targeted the PD-L1/PD-1 immunological checkpoint to induce apoptosis and anti-inflammatory response by downregulating SOD2, PARP, BCL2, SET, NF-κB, and TGF-ß. In addition, the study provided a feasible immunochemotherapeutic strategy for resistant cancers.86
In a study conducted by Cochran and Kornbluth, EVs were isolated from the NK cell line NK3.3 and their contents have been defined. It was determined that the relevant EVs contained cytolytic proteins such as perforin, granzymes A and B, granulysin, and miRNAs with anti-tumor activity. The cytotoxic functions of the relevant molecules were demonstrated in vivo on K562, Jurkat, MDA-MB-231, and MCF7 cancer cell lines and in mice injected with MDA-MB-231 cells.87 In a similar study, EVs derived from the NK3.3 cell line were administered to multiple myeloma cancer lines, and a cytotoxic effect was obtained.88 In vitro studies demonstrating the cytotoxic effect of NK-derived EVs/Exos are shown in Table 1.
Table 1.
In vitro studies demonstrating cytotoxic effect of NK-derived EVs/Exos
|
Type of NK-EVs
|
Isolation Method
|
Cancer Cell Lines
|
Effects and Results
|
Study Team
|
| Donor (PBMC) derived NK-EVs |
Ultracentrifugation |
Jurkat, K562, DAUDI,
SKBR3, 501mel |
NKEXO exert cytotoxic activity against tumors of different histologies and being ineffective against resting cells |
Reported by Lugini and colleagues.89 |
| Donor (PBMC) derived NK-EVs |
ExoQuick,
Ultracentrifugation,
Precipitation |
NALM-6; SUPB15, CHLA-136; CHLA-255 and MCF-7 |
Cancer cytotoxicity was observed by applying aNK EVs with efficient cytotoxic activity to various cancer cell lines after various scale-up isolation procedures. |
Reported by Jong and colleagues.83 |
| NK-92MI cells derived exosomes |
Ultracentrifugation and DG-UC |
B16F10 /effluc cells |
Exosomes derived from NK cells exert cytotoxic effects on melanoma cells. |
Reported by Zhu and colleagues.47 |
| Donor (PBMC) derived NK-EVs |
Filtered with 0.8-μm pore size membranes, filtrated with 50% sterile PEG8000, centrifugation. |
CHLA255, SupB15 or NAML-6 |
Multiple killing mechanisms are mediated by NK-derived EVs (caspase-independent and -dependent cell death pathways). PFN, GzmA, GzmB and GNLY protein levels are related to NK-EVs to induce cytotoxicity. |
Reported by Wu and colleagues.46 |
| Donor (PBMC) derived NK-exosome |
Differential centrifugations |
NALM-18 and K562 |
DNAM1 collaborate with other factors in NK exosome cytotoxicity possible use of NK exosomes as a therapeutic tool in cancer treatment. |
Reported by Di Pace and colleagues. 90 |
| PBMC derived - NKLs-EVs |
Differential centrifugations, ultracentrifugation |
HepG2, SW-620, MKN-74, MCF-7, T98G, |
Fas ligand, TRAIL, NKG2D, β-actin, and fibrinogen, as effector candidates based on the proteomic analysis and functional study. |
Reported by Choi and colleagues.53 |
| NK92 derived-Exos-entrapped PTX (PTX-NK-Exos) |
Ultra-high speed
differential centrifugation |
MCF-7 |
The PTX-NK-Exos drug loading system at the same dose had a higher inhibition rate on human breast cancer MCF-7 cells compared with free PTX. NK-exosomes can significantly inhibit the tumor growth. When Taxol was carried by NK-exosomes, low-dose taxol has a better antitumor effect. |
Reported by Han and colleagues.91 |
| NK3.3 and NK92 derived EVs |
Differential ultracentrifugation |
K562, Jurkat, MCF7 and MDA-MB-231 cells |
It has been determined that EVs contain cytolytic proteins such as perforin, granzymes A and B, and granulysin, and miRNAs with anti-tumor activity. The cytotoxic functions of the relevant molecules have been demonstrated on K562, Jurkat, MDA-MB-231, MCF7 cancer cell lines. |
Reported by Cochran and Kornbluth.87 |
| Patient-derived NK cell isolation |
Magnetic beads (ExoBead) and ultracentrifugation |
In-house patient-derived expanded CTC line |
Patients with non-small cell lung cancer presented with high numbers of NK and NK cell-derived exosomes compared with healthy donors. Versatile microfluidic platform can provide a foundation to be used for hitherto undiscovered roles of exosomes in cancer and other disease states. |
Reported by Kang and colleagues.92 |
| Normoxic or hypoxic (hypoxic NK92-Exo and hypoxic NK92-hIL-15-Exo) |
Ultracentrifugation |
MCF-7 and A2780 |
After hypoxic treatment, NK92-Exo exhibited significantly increased cytotoxicity, enhanced inhibition of cell proliferation. Hypoxia-treated NK92-Exo and NK92-hIL-15-Exo showed increased expression of three functional proteins of NK cells. |
Reported by Jiang and colleagues.93 |
| The NK and NK92MI was transduced to express BCL-2 siRNAs. |
Exosome Purification kit (Cell Guidance Systems, Cambridge, UK) |
HEK293T, K562, MCF-7, MEC-1, SKBR3, MCF-10A, T-47D, and MDA-MB-231 |
Specific siRNAs can be lentivirally introduced in NK cells. Then naturally loaded into exosomes, it can induce apoptosis in breast cancer cells. siBCL-2 NK Exos might serve as a new tool for cancer therapy. |
Reported by Kaban and colleagues.94 |
| NK-EV |
|
HEK293 and MSC cells |
Directly induces tumor apoptosis and promotes the action of CTL via multiplex pathways, such as up-regulating the MHC-I expression on tumor cells, reprogramming tumor-associated macrophages from pro-tumoral M2 phenotypes to tumoricidal M1 phenotypes. Tumor repression. |
Reported by Nie and colleagues.31 |
| Primary NK cells or the NK cell lines NK-92 |
Total Exosome Isolation Reagent and SEC |
HCT116, HCT-15, DU145, PC3, SK-BR-3, T-4D7, OVCAR-3, WM9, and U87 |
EV output is similar under resting or cytokine-activating conditions. EVs derived from either primary NK cells, or the NK-92 cell lines have similar abilities to induce tumor apoptosis. IL-12/15/18 cytokines-stimulated NK-EVs could be used as cancer therapy. |
Reported by Aarsund and colleagues.37 |
| PBMC derived NK-exosome |
Ultracentrifugation |
HEK-293, human epithelial kidney cells and the Raji B lymphoblast cells |
NK-EVs affect monocyte and moDCs function.
Nanoparticle-delivered NK-EV microRNAs partially recapitulate NK-EV effects in mice. |
Reported by Dosil and colleagues.50 |
| IL-15 + IL-21 stimulated NK92 derived NK-EVs |
Ultracentrifugation |
K562, Jurkat, A549, HeLa cell lines |
The cytotoxic capacity of NK-EVs stimulated with the combination of IL-15 + IL-21 increased against cancer cells.
The activating receptor CD226 (DNAM-1) on NK-EVs was enhanced by IL stimulation and cytotoxic activity was reduced after blocking CD226 with antibodies. |
Reported by Enomoto and colleagues.82 |
| Light-activatable silencing NK-derived exosomes |
Ultracentrifugation |
HepG2-Luc cells |
ROS generation upon laser irradiation triggers substantial photodynamic therapy effect and boosts M1 tumor-associated macrophages polarization and DC maturation in the tumor microenvironment. siRNAs targeting PLK1 or PD-L1 induce robust gene silencing in cancer cells, and downregulation of PD-L1 restores the immunological surveillance of T cells in tumor microenvironment |
Reported by Zhang and colleagues.95 |
| NK generation from whole blood and NK-EV isolation |
PEG precipitation and Differential centrifugation |
MCF7 |
NK cells and NK-derived EVs are promising candidates that require further experimentation to enhance their cytotoxicity. |
Reported by Parsonidis and colleagues.96 |
| NK92-MI cells derived EVs |
SEC |
K562 cells |
A novel biomanufacturing workflow with improved viability and cytotoxicity was established to produce clinical-grade NK cells using a closed-loop HFB system under SF/XF and feeder-free conditions. |
Reported by St-Denis-Bissonnette and colleagues.97 |
| NK3.3-LTV derived EVs |
A modified PEG-acetate precipitation protocol |
ARD, ARP, MER, RPMI-8226, 8226/Luc, 8226/LR5, 8226/B25, U266, and K562 |
NK3.3-LTV EVs target both sensitive and drug-resistant MM cell lines as well as primary patient MM cells in vitro, decreasing proliferation and inducing apoptotic cell death. |
Reported by Matchett and Kornbluth.88 |
| Expanded natural killer cells (eNK-EXO) |
Differential ultracentrifugation |
SKOV3, COC1/DDP and ISOE80 cells |
Deliver and enhance the killing effect of cisplatin on OC cells, reverse the immunosuppression of NK cells and gain anti-tumor activity. |
Reported by Luo and colleagues.42 |
| PBMCs derived NK-exo include Let-7b-5p |
Ultracentrifugation |
PANC-1 and NALM-18 B- |
Induction of cell death and the downregulation of cell cycle genes by let-7b-5p transfer could represent two mechanisms by which NK exosomes counteract tumor growth. Alterations in the miRNA cargo of NK-exo may represent a novel strategy exerted by cancer to evade immune response. |
Reported by Di Pace and colleagues.98 |
| NK92-MI cells derived NK-EVs |
SEC and ultrafiltration |
K562, MDA-MB-231 |
The first study to support the utility of a reliable, highly sensitive resazurin phenoxazine-based cell viability assay compared to other commonly used potency assays (e.g., flow cytometry, cell counting, LDH release assay, DNA-binding reporter assay, and confluence assay) to measure the cytotoxicity of NK-EVs against cancer cell models. |
Reported by St-Denis-Bissonnette and colleagues.99 |
| IL-15 stimulated NK92 derived NK-EVs |
Differential ultracentrifugation |
H1975-R |
NK-EVs target the PD-L1/PD-1 immunological checkpoint to induce apoptosis and anti-inflammatory response by downregulation of SOD2, PARP, BCL2, SET, NF-κB, and TGF-ß. A feasible immunochemotherapeutic strategy for refractory cancers is presented. |
Reported by Nathani and colleagues.86 |
NK, natural killer; aNK, activated NK; EVs, extracellular vesicles; DG-UC, density gradient ultracentrifugation; PBMC, peripheral blood mononuclear cell; SEC, size-exclusion chromatography; PEG, Polyethylene glycol; LDH, lactate dehydrogenase; moDCs, monocyte-derived dendritic cells; effluc, enhanced firefly luciferase.
Proteome analysis of NK EVs
With the increasing interest in EVs derived from NK cells, the underlying mechanisms of antitumor effects have become a matter of curiosity. In a study in which proteome analysis was performed, NK-EVs were isolated from human PBMC and their cytotoxic effects on human hepatocarcinoma (HEPG2), colon cancer (SW-620), stomach cancer (MKN-74), breast cancer (MCF-7) and brain cancer (T98G) were investigated. The obtained EVs were characterized by two-dimensional electrophoresis and pathway analyses, and a functional study was performed. The proteome analysis of several molecules such as Fas ligand, TRAIL, NKG2D, β-actin, and fibrinogen and functional studies identified NK-EVs as effector candidates.53 In vivo experiments showed that tumor size and mass decreased significantly compared to the control group. As seen in many sample studies above, EVs obtained from NK cells inhibit tumor cells. One study aimed to obtain an NK EV subpopulation that was thought to be more successful in targeting solid tumors. Within the scope of the relevant study, EVs obtained from NK92 cells were first fractionated by DG-UC or SEC. Subsequent proteomic analyses yielded an EV subpopulation carrying the cytolytic granule marker natural killer granule protein 7 (NKG7) enriched with cytolytic proteins.100
Immunotherapeutic effect of NK-Exos
In a study conducted in 2012, exosomes containing typical protein markers and killer proteins (i.e., Fas ligand and perforin molecules) were derived from non-activated and activated NK cells isolated from healthy donor blood (PBMC) and the cytotoxic effect of these exosomes on Jurkat (T-cell leukemia), K562 (erythroleukemia), DAUDI (Burkitt lymphoma) (all obtained from American Type Cell Culture), SKBR3 (metastatic breast adenocarcinoma) and 501mel (metastatic melanoma cell line) was observed. It was found that the isolated exosomes were taken up by tumor cells but not by healthy cells.89 The results demonstrate that the exosomes used selectively target cancer cells without affecting normal cells. This method could soon lead to developing specialized therapies in this field. In another study using PBMC as a source of NK cells, the effects of exosomes on neuroblastoma (NB) tumor cells obtained from NK cells previously exposed to NB tumor cells, exosomes obtained from naive NK cells, and exosomes derived from NB were observed. It has been determined that exosomes obtained from tumor cells act in a way that supports the tumor niche. Exosomes derived from NKs previously exposed to NB have been shown to activate resting NKs and train NKs to increase the NK-mediated anti-NB tumor reaction.52
A different approach was taken in a study by Zhu L et al. A B16F10/efflux cell xenograft model was used to determine the immunotherapeutic effect of exosomes obtained from the NK92MI cell line. After the injection of exosomes into the tumor, tumor growth was monitored with the IVIS Lumina imaging system and ultrasound. Western blot analyses confirmed that exosomes contained two functional proteins, FasL and perforin, and FasL membrane expression was determined. As a result, no side effects were observed from exosomes administered to control groups, while in vitro antitumor effect of NK-92 Exo against B16F10/efflux cells was detected.47
In another study by Zhu et al in 2018, “exosome mimics” were obtained due to the difficulty of purifying exosomes. Using the extrusion method, nanovesicles were developed by passing NK92-MI cells through filters with gradually decreasing pore sizes. The obtained exosome mimics were applied to cancer cells (glioblastoma, breast carcinoma, anaplastic thyroid cancer and hepatic carcinoma) and their cytotoxicity was evaluated using bioluminescence imaging and CCK-8 assay. As a result, a decrease in cell survival markers and increased apoptosis markers were detected.101
In addition to studies where exosomes are applied without modification, there are different approaches where their liposome integration is performed. In a study conducted on the relevant subject, a biomimetic nanostructure was designed from NK cell membrane-infused fusogenic liposome (NKsome) for tumor-targeted drug delivery. In this method, activated NK cell membrane containing receptor proteins was isolated from NK-92 cells and co-extruded with fusogenic liposomes to form NKsomes. The in vitro tumor-targeting ability of the fusogenic NKsome has been investigated against normal human osteoblast (NHost) and human breast cancer cells (MCF-7). In addition, the anti-tumor activity against MCF-7-induced solid tumor model in NU/NU mice was investigated by packaging the chemotherapeutic drug doxorubicin in the NKsome. Promising anti-tumor effects to have been observed in in vivo studies. 102
In a study conducted by Wang et al in 2019, NK-EXO and NN/NK-EXO (biomimetic core-shell nanoparticles - NNs) samples from PB-NK of healthy donors were applied to human breast cancer (MDA-MB-231) and NB (CHLA-255) cells at 10, 20 or 40 µg amounts. It was found that NK-EXOs reduced the viability of both tumor cell lines in a dose-dependent manner. NN/NK-EXOs exhibited higher levels of tumor cytotoxicity compared to NK-EXOs. In the in vivo study, NN/NK-EXOs provided better targeting efficiency and more significant suppression of tumor growth compared to NK-EXOs.103 (Table 2)
Table 2.
In vivo studies demonstrating cytotoxic effect of NK-derived EVs/Exos
|
Type of NK-EVs and dosage
|
Cancer cell lines and type of animals
|
Effects and results
|
Study team
|
| 20 µg/100 µL of human NK-EXO |
B16F10/effluc cells injected to 6-week-old female C57BL/6 mice (n: 6) |
Tumor mass was efficiently reduced in the treatment group. |
Reported by Zhu and colleagues.47 |
| 100 µg/150 µL (IV) and 30 µg/50 µL (IT) NK-EM (3 times at intervals of 3 days) |
D54 cells (5 × 106 cells/100 µL) injected to 6-week-old female BALB/c mice (n: 5) |
It was shown to better reduce tumor mass than IV in the treatment group. |
Reported by Zhu and colleagues.101 |
| 1 mg/kg/d of miR-186 enriched human NK-EVs (3 times per week) |
CHLA-136-Fluc cells (1 × 106 cells) injected to 4- to 8-week-old female and male NSG mice (n: 5-10) |
Tumor cells were decreased in the animals that received miR-186-enriched NK-EVs compared to the control group. Survival rate was improved. |
Reported by Neviani and colleagues.45 |
| 100 µg of human NK-EXO (with or without NN let-7a loaded polyamidoamine dendrimer) |
CHLA-255-luc cells (1 × 107 cells/500 µL) injected to 6-week-old female NOD/SCID mice (n: 3) |
Suppression of tumor growth was better on the NN/NK-EXOs compared to the NK-EXOs. |
Reported by Wangand colleagues.103 |
| 50 µg of human NK-EVs / 50 µg of human NK-EVsIL-15 (5 times at intervals of 2 days) |
U87/MG/F cells injected to 6-week-old female BALB/c nude mice (n: 15) |
Tumor weight was significantly reduced on both groups. Also, NK-EVsIL-15 were significantly more effective than NK-EVs. |
Reported by Zhu and colleagues.81 |
| 50 µg/100 µL of human NK-EVs (3 times weekly) |
MCF-7 cells (3 × 106 cells) injected to 5-week-old, female athymic nude mice (n: 5) |
Tumor weight reduced significantly compared to the control group. |
Reported by Choi and colleagues.53 |
| 50 µg of human NK3.3-EVs (7 times at intervals of 3 to 4 days) |
MDA-MB-231 cells (2 × 106 cells) injected to female athymic nude mice (n: 4-5) |
NK3.3 EVs inhibited proliferation and induced caspase-mediated apoptosis and cell death. |
Reported by Cochran and Kornbluth.87 |
| 50, 100, 200 or 500 µg of human NK-EXO (6 times at intervals of 2 days) |
Hep3B cells (1 × 107 cells/100 µL) injected to the right back and (2 × 106 cells/50 µL) liver of 6-week-old male BALB/c nude mice respectively (n: 5). |
NK-exo exhibited the active targeting ability and potent therapeutic effects in orthotopic and subcutaneous HCC in vivo models. |
Reported by Kim and Min.85 |
| NK-EVs 200 µg, CBP 25 mg/kg, NK-EVs 200 µg + CBP 25 mg/kg (10 days) |
H1975R tumor cells xenografts |
Tumor regression has been observed in in vivo models. |
Reported by Nathani and colleagues.86 |
effluc, enhanced firefly luciferase
In a 2020 study, human NK cells isolated from PBMC were cultured with IL-2 and IL-15, and then exosomes obtained from the culture were characterized. Analysis of molecules present or released on the exosome surface identified molecules that play important roles in NK cell function, including IFN-γ, lymphocyte function associated antigen (LFA-1), DNAX accessory molecule-1 (DNAM1), and programmed cell death protein (PD-1). The relevant exosomes showed dose-dependent cytotoxic effects on human childhood B acute lymphoblastic leukemia (NALM-18) and erythroleukemia (K562) cancer cell lines. Moreover, in experiments where DNAM1 and its ligand were inactivated with antibodies, the relevant molecule was found to play a role in exosome-mediated cytotoxicity. Possible future therapeutic roles of exosomes have been highlighted.90
NK EVs and NK Exos as a carrier
The method of delivering various chemotherapeutic agents and miRNAs to the tumor microenvironment with liposomes or nanoparticles can be put into practice today and has been mentioned in many studies.104,105 EVs, just like liposomes and nanoparticles, have the potential to be used as carriers, and studies on the subject are increasing day by day.106 The success of EVs in infiltrating the tumor microenvironment has led to the idea of applying them as carriers in the treatment of solid tumors. In addition, the biocompatibility of EVs supports this idea, and successes have been achieved in previous in vitro and in vivo studies on the subject.107,108
In a study conducted with NK cell-derived exosomes, exosomes loaded with the chemotherapeutic agent cisplatin showed cytotoxic effects on ovarian cancer cells and increased the antitumor effect of NK cells.42 In another study, sorafenib was applied to triple-negative breast cancer cells via EV-derived exosomes, and death was triggered by apoptosis in the cancer cells.109
In another study using NK-derived exosomes as chemotherapeutic agent carriers, NK exosomes were modified to contain the agent paclitaxel. The created structure was found to inhibit the proliferation of MCF-7 cells and cause apoptosis of tumor cells.91
In addition to chemotherapeutic agents, miRNAs are used in cancer treatment applications.110 A study has shown that miRNA let-7b-5p, enriched in NK exosomes, has been found to have an antitumor effect on pancreatic cancer.98 In another study, NK-derived exosomes were enriched with miR-186 and applied to tumor cells. Researchers have shown that the generated constructs down-regulate the expression of aurora A kinase (AURKA) and v-myc avian myelocytomatosis viral oncogene NB-derived homolog (MYCN) in in vivo models, thereby suppressing cell proliferation and inducing apoptosis in NB cells45 (Table 2).
In a study investigating the immunomodulatory aspect of NK-EVs, miRNAs associated with EVs derived from NK cells obtained from PBMC were identified and characterized by next-generation sequencing. As a result, it was determined that NK-EVs support Th1 polarization and activation of monocytes and DCs. NK cell function was attempted to be resolved.50 Researchers also found that miR-92a and miR-155 increase IFN-γ production.50 In a study to enhance the immunomodulatory activity of NK cell-derived exosomes, Light-activatable silencing NK-derived exosomes (LASNEO) were engineered with hydrophilic small interfering RNA (siRNA) and hydrophobic photosensitizer Ce6. Profiles of genes involved in the apoptosis pathway of exosome-treated cells were performed using Western blot and RNA-seq, revealing that NEO elicits effective NK cell-like cytotoxicity against tumor cells. In this method, the production of reactive oxygen species (ROS) upon laser irradiation not only triggered a significant photodynamic therapy effect but also provided an immunomodulatory effect in the tumor microenvironment. 95
In a study, a different approach was followed, and NK92MI, a well-known NK cell line, was genetically modified to target BCL-2. NK92MI was transformed by lentiviral to express BCL-2 siRNAs (siBCL-2) in exosomes (NKExos) and then evaluated for its potential to treat ER + breast cancer.94
Production methods of NK EVs and NK Exos
In addition to the pure isolation of EVs intended for use in immunotherapy, obtaining enough product for treatment is one of the important factors affecting the success of the treatment. In a study evaluating the effect of NK EVs on breast cancer cells, the relevant situation was emphasized as an important point for treatment success.96 In a recent study, a new biomanufacturing workflow using a closed-circuit hollow fiber bioreactor was invented to continuously produce NK-EVs from the NK92-MI cell line under serum-free, Xeno-free, and feeder-free conditions.97
Suppose the large-scale production methods of NK EVs are to be classified generally. In that case, they can be classified as follows:
-
Establishment of high-capacity “cell factories” based on the natural production of EVs,
-
Induction of EV production under cell stressors,
-
Formation of biomimetic vesicles based on cell fragmentation.111
Bioreactors are widely used to establish high-capacity cell factories. Although the relevant systems are advantageous regarding “efficient use of space” and “conservation of media,” restrictive effects such as cell aging and spontaneous cell differentiation may occur.112 Chemical agents such as “Sulfhydryl-blocking agents” or physical stimulation applications such as “Acoustic treatment” or “thermal stress” can stimulate EV production with cell stressors.113-115 Methods such as ultrasound homogenization, nitrogen cavitation, extrusion, and cell membrane cutting can be used to obtain biomimetic vesicles.70,116-118
In a study published in 2021, a microfluidic system was invented to collect patient-specific NK cells and the biogenesis of NK exosomes on a chip. The relevant study was based on a patient- specific NK-based immunotherapeutic approach.92
A study was conducted to evaluate the number of exosomes released by a subset of NK cells under hypoxic conditions and their immunotherapeutic effect on cancer cells. NK92 exosomes were characterized under normoxic and hypoxic conditions. Then, various experiments were performed to determine cell proliferation, cytotoxicity, and apoptosis. After 48 hours of hypoxic exposure, increased cytotoxicity and decreased cell proliferation were detected. Increased expression of FasL, perforin, and granzyme B was detected in exosomes under hypoxic conditions. Thus, it has been suggested that this method, which also supports the increase of NK exosomes, is promising for cancer immunotherapy.93
Determination of NK EVs cytotoxicity
Standardized potency tests are needed for reliable assessment of NK-EV cytotoxicity. In a study conducted for this purpose, the resazurin phenoxazine-based cell viability potency test (measurement of cellular redox metabolism) was comprehensively evaluated to measure the cytotoxicity of NK-EVs on K562 leukemia cells and MDA-MB-231 breast cancer cells in vitro. Analytical parameters such as specificity, selectivity, accuracy, precision, linearity, range, and stability were considered during the evaluation. The results showed that the relevant resazurin-based cell viability potency assay reproducibly and reliably measured the dose-response ratio of the cytotoxic activity of NK-EVs in both cancer models. The dose-response of NK-EVs for cytotoxicity showed a strong correlation (| ρ | ∼ 0.8) with the levels of cytotoxic factors (FasL, GNLY, GzmB, PFN and IFN-γ) of NK-EVs. Thus, the accuracy of the test was demonstrated. In addition, the cytotoxicity changes of degraded NK-EVs were also detected with this assay, and the potential loss of sample integrity could be determined. The relevant assay was emphasized as “a high-throughput and quantitative method”.99
Conclusion
The therapeutic potential of NK-EVs represents a novel and innovative development in cancer immunotherapy. While traditional treatment strategies such as chemotherapy, radiation therapy, and surgery are still used, their limitations in addressing drug resistance, metastasis, and tumor recurrence reveal the need for pioneering and innovative approaches. NK cells, with their ability to target and selectively destroy cancer cells and not damage healthy cells, can be a promising alternative. In recent years, NK-EVs, as natural carriers of bioactive molecules, have played a prominent role as versatile tools for cancer treatment. These vesicles can deliver therapeutic agents more effectively to tumor sites and overcome many challenges associated with treatments that are used daily.
The cytotoxic capabilities that they show and the immunomodulatory effects that they have make NK-EVs have the potential to be used in the treatment of various cancers, including solid tumors and hematological malignancies, which can create new opportunities to increase the results of cancer treatment. By delivering chemotherapeutic agents, miRNAs and siRNAs, NK-EVs can easily enable precise and targeted therapies, reduce unwanted and off-target side effects, and improve treatment efficacy. Despite recent encouraging results, challenges such as large-scale production, sustainability, and targeted delivery of NK-EVs remain. Advances in bioreactor systems, isolation techniques, and genetic engineering provide solutions to these challenges and pave the way for clinical application.
Future research should focus more on optimizing the production of these NK-EVs, increasing our knowledge of their mechanism of action at the molecular level. Future research should focus on combining them with other immunological methods, such as checkpoint inhibitors or CAR-T cells. The clinical translation of NK-EVs to routine cancer treatment will depend on the continuous improvement of their production processes, the evaluation of the safety of this therapeutic method, and the therapeutic efficacy. By overcoming these obstacles, NK-EVs have the potential to revolutionize cancer treatment and provide patients with more effective, personalized, and less toxic treatment options in the fight against cancer.
Competing Interests
The authors declare no conflicts of interest.
Ethical Approval
Not applicable.
References
- Maloney SM, Hoover CA, Morejon-Lasso LV, Prosperi JR. Mechanisms of taxane resistance. Cancers (Basel) 2020; 12(11):3323. doi: 10.3390/cancers12113323 [Crossref] [ Google Scholar]
- Das T, Anand U, Pandey SK, Ashby CR, Jr Jr. , Assaraf YG, Chen ZS, et al Therapeutic strategies to overcome taxane resistance in cancer. Drug Resist Updat 2021; 55:100754. doi: 10.1016/j.drup.2021.100754 [Crossref] [ Google Scholar]
- Francolini G, Frosini G, Di Cataldo V, Detti B, Carnevale MG, Banini M. Predictive factors for tolerance to taxane based chemotherapy in older adults affected by metastatic prostate cancer (ANCHISES-NCT05471427): a prospective observational trial including patients with metastatic hormone sensitive and castrate resistant prostate cancer treated with taxane chemotherapy. J Geriatr Oncol 2023; 14(1):101411. doi: 10.1016/j.jgo.2022.11.010 [Crossref] [ Google Scholar]
- Tucci M, Zichi C, Buttigliero C, Vignani F, Scagliotti GV, Di Maio M. Enzalutamide-resistant castration-resistant prostate cancer: challenges and solutions. Onco Targets Ther 2018; 11:7353-68. doi: 10.2147/ott.S153764 [Crossref] [ Google Scholar]
- Tan S, Li D, Zhu X. Cancer immunotherapy: pros, cons and beyond. Biomed Pharmacother 2020; 124:109821. doi: 10.1016/j.biopha.2020.109821 [Crossref] [ Google Scholar]
- Wu F, Xie M, Hun M, She Z, Li C, Luo S. Natural killer cell-derived extracellular vesicles: novel players in cancer immunotherapy. Front Immunol 2021; 12:658698. doi: 10.3389/fimmu.2021.658698 [Crossref] [ Google Scholar]
- Poli A, Michel T, Thérésine M, Andrès E, Hentges F, Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology 2009; 126(4):458-65. doi: 10.1111/j.1365-2567.2008.03027.x [Crossref] [ Google Scholar]
- Chen Y, Lu D, Churov A, Fu R. Research progress on NK cell receptors and their signaling pathways. Mediators Inflamm 2020; 2020:6437057. doi: 10.1155/2020/6437057 [Crossref] [ Google Scholar]
- Sivori S, Vacca P, Del Zotto G, Munari E, Mingari MC, Moretta L. Human NK cells: surface receptors, inhibitory checkpoints, and translational applications. Cell Mol Immunol 2019; 16(5):430-41. doi: 10.1038/s41423-019-0206-4 [Crossref] [ Google Scholar]
- Voskoboinik I, Whisstock JC, Trapani JA. Perforin and granzymes: function, dysfunction and human pathology. Nat Rev Immunol 2015; 15(6):388-400. doi: 10.1038/nri3839 [Crossref] [ Google Scholar]
- Prager I, Watzl C. Mechanisms of natural killer cell-mediated cellular cytotoxicity. J Leukoc Biol 2019; 105(6):1319-29. doi: 10.1002/jlb.Mr0718-269r [Crossref] [ Google Scholar]
- Liu S, Galat V, Galat Y, Lee YKA, Wainwright D, Wu J. NK cell-based cancer immunotherapy: from basic biology to clinical development. J Hematol Oncol 2021; 14(1):7. doi: 10.1186/s13045-020-01014-w [Crossref] [ Google Scholar]
- Xie G, Dong H, Liang Y, Ham JD, Rizwan R, Chen J. CAR-NK cells: a promising cellular immunotherapy for cancer. EBioMedicine 2020; 59:102975. doi: 10.1016/j.ebiom.2020.102975 [Crossref] [ Google Scholar]
- Shin MH, Oh E, Kim Y, Nam DH, Jeon SY, Yu JH. Recent advances in CAR-based solid tumor immunotherapy. Cells 2023; 12(12):1606. doi: 10.3390/cells12121606 [Crossref] [ Google Scholar]
- Gong Y, Klein Wolterink RG, Wang J, Bos GM, Germeraad WT. Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. J Hematol Oncol 2021; 14(1):73. doi: 10.1186/s13045-021-01083-5 [Crossref] [ Google Scholar]
- Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 2014; 30:255-89. doi: 10.1146/annurev-cellbio-101512-122326 [Crossref] [ Google Scholar]
- Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 1983; 33(3):967-78. doi: 10.1016/0092-8674(83)90040-5 [Crossref] [ Google Scholar]
- Harding C, Heuser J, Stahl P. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. Eur J Cell Biol 1984; 35(2):256-63. [ Google Scholar]
- György B, Szabó TG, Pásztói M, Pál Z, Misják P, Aradi B. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci 2011; 68(16):2667-88. doi: 10.1007/s00018-011-0689-3 [Crossref] [ Google Scholar]
- Sandvig K, Llorente A. Proteomic analysis of microvesicles released by the human prostate cancer cell line PC-3. Mol Cell Proteomics 2012;11(7):M111.012914. 10.1074/mcp.M111.012914.
- Yoshioka Y, Konishi Y, Kosaka N, Katsuda T, Kato T, Ochiya T. Comparative marker analysis of extracellular vesicles in different human cancer types. J Extracell Vesicles 2013; 2:20424. doi: 10.3402/jev.v2i0.20424 [Crossref] [ Google Scholar]
- Tetta C, Ghigo E, Silengo L, Deregibus MC, Camussi G. Extracellular vesicles as an emerging mechanism of cell-to-cell communication. Endocrine 2013; 44(1):11-9. doi: 10.1007/s12020-012-9839-0 [Crossref] [ Google Scholar]
- Chaput N, Théry C. Exosomes: immune properties and potential clinical implementations. Semin Immunopathol 2011; 33(5):419-40. doi: 10.1007/s00281-010-0233-9 [Crossref] [ Google Scholar]
- Burton JB, Carruthers NJ, Stemmer PM. Enriching extracellular vesicles for mass spectrometry. Mass Spectrom Rev 2023; 42(2):779-95. doi: 10.1002/mas.21738 [Crossref] [ Google Scholar]
- El Andaloussi S, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov 2013; 12(5):347-57. doi: 10.1038/nrd3978 [Crossref] [ Google Scholar]
- Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP. Isolation of extracellular vesicles: general methodologies and latest trends. Biomed Res Int 2018; 2018:8545347. doi: 10.1155/2018/8545347 [Crossref] [ Google Scholar]
- Stam J, Bartel S, Bischoff R, Wolters JC. Isolation of extracellular vesicles with combined enrichment methods. J Chromatogr B Analyt Technol Biomed Life Sci 2021; 1169:122604. doi: 10.1016/j.jchromb.2021.122604 [Crossref] [ Google Scholar]
- Federici C, Shahaj E, Cecchetti S, Camerini S, Casella M, Iessi E. Natural-killer-derived extracellular vesicles: immune sensors and interactors. Front Immunol 2020; 11:262. doi: 10.3389/fimmu.2020.00262 [Crossref] [ Google Scholar]
- Farcas M, Inngjerdingen M. Natural killer cell-derived extracellular vesicles in cancer therapy. Scand J Immunol 2020; 92(4):e12938. doi: 10.1111/sji.12938 [Crossref] [ Google Scholar]
- Veerman RE, Güçlüler Akpinar G, Eldh M, Gabrielsson S. Immune cell-derived extracellular vesicles - functions and therapeutic applications. Trends Mol Med 2019; 25(5):382-94. doi: 10.1016/j.molmed.2019.02.003 [Crossref] [ Google Scholar]
- Nie W, Fan W, Jiang A, Wu G, Liu H, Huang LL. Natural killer cell-derived extracellular vesicle significantly enhanced adoptive T cell therapy against solid tumors via versatilely immunomodulatory coordination. Sci China Chem 2021; 64(11):1999-2009. doi: 10.1007/s11426-021-1085-8 [Crossref] [ Google Scholar]
- Si C, Gao J, Ma X. Natural killer cell-derived exosome-based cancer therapy: from biological roles to clinical significance and implications. Mol Cancer 2024; 23(1):134. doi: 10.1186/s12943-024-02045-4 [Crossref] [ Google Scholar]
- Sakamoto N, Ishikawa T, Kokura S, Okayama T, Oka K, Ideno M. Phase I clinical trial of autologous NK cell therapy using novel expansion method in patients with advanced digestive cancer. J Transl Med 2015; 13:277. doi: 10.1186/s12967-015-0632-8 [Crossref] [ Google Scholar]
- Fang F, Xie S, Chen M, Li Y, Yue J, Ma J. Advances in NK cell production. Cell Mol Immunol 2022; 19(4):460-81. doi: 10.1038/s41423-021-00808-3 [Crossref] [ Google Scholar]
- Shah NN, Baird K, Delbrook CP, Fleisher TA, Kohler ME, Rampertaap S. Acute GVHD in patients receiving IL-15/4-1BBL activated NK cells following T-cell-depleted stem cell transplantation. Blood 2015; 125(5):784-92. doi: 10.1182/blood-2014-07-592881 [Crossref] [ Google Scholar]
- Gong JH, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia 1994; 8(4):652-8. [ Google Scholar]
- Aarsund M, Segers FM, Wu Y, Inngjerdingen M. Comparison of characteristics and tumor targeting properties of extracellular vesicles derived from primary NK cells or NK-cell lines stimulated with IL-15 or IL-12/15/18. Cancer Immunol Immunother 2022; 71(9):2227-38. doi: 10.1007/s00262-022-03161-0 [Crossref] [ Google Scholar]
- Boyd-Gibbins N, Karagiannis P, Hwang DW, Kim SI. iPSCs in NK cell manufacturing and NKEV development. Front Immunol 2022; 13:890894. doi: 10.3389/fimmu.2022.890894 [Crossref] [ Google Scholar]
- Kundu S, Gurney M, O’Dwyer M. Generating natural killer cells for adoptive transfer: expanding horizons. Cytotherapy 2021; 23(7):559-66. doi: 10.1016/j.jcyt.2020.12.002 [Crossref] [ Google Scholar]
- Hu W, Wang G, Huang D, Sui M, Xu Y. Cancer immunotherapy based on natural killer cells: current progress and new opportunities. Front Immunol 2019; 10:1205. doi: 10.3389/fimmu.2019.01205 [Crossref] [ Google Scholar]
- Fabbri M. Natural killer cell-derived vesicular miRNAs: a new anticancer approach?. Cancer Res 2020; 80(1):17-22. doi: 10.1158/0008-5472.Can-19-1450 [Crossref] [ Google Scholar]
- Luo H, Zhou Y, Zhang J, Zhang Y, Long S, Lin X. NK cell-derived exosomes enhance the anti-tumor effects against ovarian cancer by delivering cisplatin and reactivating NK cell functions. Front Immunol 2022; 13:1087689. doi: 10.3389/fimmu.2022.1087689 [Crossref] [ Google Scholar]
- Tao B, Du R, Zhang X, Jia B, Gao Y, Zhao Y. Engineering CAR-NK cell derived exosome disguised nano-bombs for enhanced HER2 positive breast cancer brain metastasis therapy. J Control Release 2023; 363:692-706. doi: 10.1016/j.jconrel.2023.10.007 [Crossref] [ Google Scholar]
- Qi Y, Zhao X, Dong Y, Wang M, Wang J, Fan Z. Opportunities and challenges of natural killer cell-derived extracellular vesicles. Front Bioeng Biotechnol 2023; 11:1122585. doi: 10.3389/fbioe.2023.1122585 [Crossref] [ Google Scholar]
- Neviani P, Wise PM, Murtadha M, Liu CW, Wu CH, Jong AY. Natural killer-derived exosomal miR-186 inhibits neuroblastoma growth and immune escape mechanisms. Cancer Res 2019; 79(6):1151-64. doi: 10.1158/0008-5472.Can-18-0779 [Crossref] [ Google Scholar]
- Wu CH, Li J, Li L, Sun J, Fabbri M, Wayne AS. Extracellular vesicles derived from natural killer cells use multiple cytotoxic proteins and killing mechanisms to target cancer cells. J Extracell Vesicles 2019; 8(1):1588538. doi: 10.1080/20013078.2019.1588538 [Crossref] [ Google Scholar]
- Zhu L, Kalimuthu S, Gangadaran P, Oh JM, Lee HW, Baek SH. Exosomes derived from natural killer cells exert therapeutic effect in melanoma. Theranostics 2017; 7(10):2732-45. doi: 10.7150/thno.18752 [Crossref] [ Google Scholar]
- Buzas EI. The roles of extracellular vesicles in the immune system. Nat Rev Immunol 2023; 23(4):236-50. doi: 10.1038/s41577-022-00763-8 [Crossref] [ Google Scholar]
- Jia R, Cui K, Li Z, Gao Y, Zhang B, Wang Z. NK cell-derived exosomes improved lung injury in mouse model of Pseudomonas aeruginosa lung infection. J Physiol Sci 2020; 70(1):50. doi: 10.1186/s12576-020-00776-9 [Crossref] [ Google Scholar]
- Dosil SG, Lopez-Cobo S, Rodriguez-Galan A, Fernandez-Delgado I, Ramirez-Huesca M, Milan-Rois P. Natural killer (NK) cell-derived extracellular-vesicle shuttled microRNAs control T cell responses. Elife 2022; 11:e76319. doi: 10.7554/eLife.76319 [Crossref] [ Google Scholar]
- Poli A, Michel T, Thérésine M, Andrès E, Hentges F, Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology 2009; 126(4):458-65. doi: 10.1111/j.1365-2567.2008.03027.x [Crossref] [ Google Scholar]
- Shoae-Hassani A, Hamidieh AA, Behfar M, Mohseni R, Mortazavi-Tabatabaei SA, Asgharzadeh S. NK cell-derived exosomes from NK cells previously exposed to neuroblastoma cells augment the antitumor activity of cytokine-activated NK cells. J Immunother 2017; 40(7):265-76. doi: 10.1097/cji.0000000000000179 [Crossref] [ Google Scholar]
- Choi JW, Lim S, Kang JH, Hwang SH, Hwang KC, Kim SW. Proteome analysis of human natural killer cell derived extracellular vesicles for identification of anticancer effectors. Molecules 2020; 25(21):5216. doi: 10.3390/molecules25215216 [Crossref] [ Google Scholar]
- Dillman RO. Cancer immunotherapy. Cancer Biother Radiopharm 2011; 26(1):1-64. doi: 10.1089/cbr.2010.0902 [Crossref] [ Google Scholar]
- Gubin MM, Schreiber RD. CANCER The odds of immunotherapy success. Science 2015; 350(6257):158-9. doi: 10.1126/science.aad4140 [Crossref] [ Google Scholar]
- Rescigno P, Fenor de la Maza MD, Burnett SM, Villacampa G, Stiles M, Cafferty FH. Phase II trial of pembrolizumab for patients suffering from metastatic castration resistant prostate cancer (mCRPC) with DNA repair defects, high tumour mutation burden, and/or high CD3 counts (PERSEUS1). J Clin Oncol 2024; 42(4 Suppl):138. doi: 10.1200/JCO.2024.42.4_suppl.138 [Crossref] [ Google Scholar]
- Dastouri M, Kilic N, Yilmaz H. The apoptotic effects of NK-92 cells stimulated with an anti-CD226 antibody on MDA-MB-231 triple-negative breast cancer cells. Med Oncol 2023; 40(8):228. doi: 10.1007/s12032-023-02080-z [Crossref] [ Google Scholar]
- Patwekar M, Sehar N, Patwekar F, Medikeri A, Ali S, Aldossri RM. Novel immune checkpoint targets: a promising therapy for cancer treatments. Int Immunopharmacol 2024; 126:111186. doi: 10.1016/j.intimp.2023.111186 [Crossref] [ Google Scholar]
- Justiz-Vaillant AA, Soodeen S, Gopaul D, Arozarena-Fundora R, Akpaka PE. The future directions of CAR-T cell therapy: unlocking the potential of immunotherapy in cancer treatment. Preprints [Preprint]. June 4, 2024. Available from: https://www.preprints.org/manuscript/202406.0155.
- Shapir Itai Y, Barboy O, Salomon R, Bercovich A, Xie K, Winter E, et al. Bispecific dendritic-T cell engager potentiates anti-tumor immunity. Cell 2024;187(2):375-89.e18. 10.1016/j.cell.2023.12.011.
- Babamohamadi M, Mohammadi N, Faryadi E, Haddadi M, Merati A, Ghobadinezhad F. Anti-CTLA-4 nanobody as a promising approach in cancer immunotherapy. Cell Death Dis 2024; 15(1):17. doi: 10.1038/s41419-023-06391-x [Crossref] [ Google Scholar]
- Lee TA, Tsai EY, Liu SH, Hsu Hung SD, Chang SJ, Chao CH. Post-translational modification of PD-1: potential targets for cancer immunotherapy. Cancer Res 2024; 84(6):800-7. doi: 10.1158/0008-5472.Can-23-2664 [Crossref] [ Google Scholar]
- Reese Z, Straubhar A, Pal SK, Agarwal N. Ipilimumab in the treatment of prostate cancer. Future Oncol 2015; 11(1):27-37. doi: 10.2217/fon.14.196 [Crossref] [ Google Scholar]
- Sharma P, Pachynski RK, Narayan V, Fléchon A, Gravis G, Galsky MD, et al. Nivolumab plus ipilimumab for metastatic castration-resistant prostate cancer: preliminary analysis of patients in the CheckMate 650 trial. Cancer Cell 2020;38(4):489-99.e3. 10.1016/j.ccell.2020.08.007.
- Stultz J, Fong L. How to turn up the heat on the cold immune microenvironment of metastatic prostate cancer. Prostate Cancer Prostatic Dis 2021; 24(3):697-717. doi: 10.1038/s41391-021-00340-5 [Crossref] [ Google Scholar]
- Ma Z, Zhang W, Dong B, Xin Z, Ji Y, Su R. Docetaxel remodels prostate cancer immune microenvironment and enhances checkpoint inhibitor-based immunotherapy. Theranostics 2022; 12(11):4965-79. doi: 10.7150/thno.73152 [Crossref] [ Google Scholar]
- Bansal D, Reimers MA, Knoche EM, Pachynski RK. Immunotherapy and immunotherapy combinations in metastatic castration-resistant prostate cancer. Cancers (Basel) 2021; 13(2):334. doi: 10.3390/cancers13020334 [Crossref] [ Google Scholar]
- Bonaventura P, Shekarian T, Alcazer V, Valladeau-Guilemond J, Valsesia-Wittmann S, Amigorena S. Cold tumors: a therapeutic challenge for immunotherapy. Front Immunol 2019; 10:168. doi: 10.3389/fimmu.2019.00168 [Crossref] [ Google Scholar]
- Martori C, Sanchez-Moral L, Paul T, Pardo JC, Font A, Ruiz de Porras V. Macrophages as a therapeutic target in metastatic prostate cancer: a way to overcome immunotherapy resistance?. Cancers (Basel) 2022; 14(2):440. doi: 10.3390/cancers14020440 [Crossref] [ Google Scholar]
- Wang X, Hu S, Li J, Zhu D, Wang Z, Cores J. Extruded mesenchymal stem cell nanovesicles are equally potent to natural extracellular vesicles in cardiac repair. ACS Appl Mater Interfaces 2021; 13(47):55767-79. doi: 10.1021/acsami.1c08044 [Crossref] [ Google Scholar]
- Geuijen C, Tacken P, Wang LC, Klooster R, van Loo PF, Zhou J. A human CD137 × PD-L1 bispecific antibody promotes anti-tumor immunity via context-dependent T cell costimulation and checkpoint blockade. Nat Commun 2021; 12(1):4445. doi: 10.1038/s41467-021-24767-5 [Crossref] [ Google Scholar]
- Heitmann JS, Pfluegler M, Jung G, Salih HR. Bispecific antibodies in prostate cancer therapy: current status and perspectives. Cancers (Basel) 2021; 13(3):549. doi: 10.3390/cancers13030549 [Crossref] [ Google Scholar]
- June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science 2018; 359(6382):1361-5. doi: 10.1126/science.aar6711 [Crossref] [ Google Scholar]
- Narayan V, Barber-Rotenberg JS, Jung IY, Lacey SF, Rech AJ, Davis MM. PSMA-targeting TGFβ-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat Med 2022; 28(4):724-34. doi: 10.1038/s41591-022-01726-1 [Crossref] [ Google Scholar]
- Peters DT, Savoldo B, Grover NS. Building safety into CAR-T therapy. Hum Vaccin Immunother 2023; 19(3):2275457. doi: 10.1080/21645515.2023.2275457 [Crossref] [ Google Scholar]
- Bryceson YT, Chiang SC, Darmanin S, Fauriat C, Schlums H, Theorell J. Molecular mechanisms of natural killer cell activation. J Innate Immun 2011; 3(3):216-26. doi: 10.1159/000325265 [Crossref] [ Google Scholar]
- Klingemann H. The NK-92 cell line-30 years later: its impact on natural killer cell research and treatment of cancer. Cytotherapy 2023; 25(5):451-7. doi: 10.1016/j.jcyt.2022.12.003 [Crossref] [ Google Scholar]
- Morgan MA, Büning H, Sauer M, Schambach A. Use of cell and genome modification technologies to generate improved “off-the-shelf” CAR T and CAR NK cells. Front Immunol 2020; 11:1965. doi: 10.3389/fimmu.2020.01965 [Crossref] [ Google Scholar]
- Shokouhifar A, Firouzi J, Nouri M, Anani Sarab G, Ebrahimi M. NK cell upraise in the dark world of cancer stem cells. Cancer Cell Int 2021; 21(1):682. doi: 10.1186/s12935-021-02400-1 [Crossref] [ Google Scholar]
- Aarsund M, Segers FM, Wu Y, Inngjerdingen M. Comparison of characteristics and tumor targeting properties of extracellular vesicles derived from primary NK cells or NK-cell lines stimulated with IL-15 or IL-12/15/18. Cancer Immunol Immunother 2022; 71(9):2227-38. doi: 10.1007/s00262-022-03161-0 [Crossref] [ Google Scholar]
- Zhu L, Kalimuthu S, Oh JM, Gangadaran P, Baek SH, Jeong SY. Enhancement of antitumor potency of extracellular vesicles derived from natural killer cells by IL-15 priming. Biomaterials 2019; 190-191:38-50. doi: 10.1016/j.biomaterials.2018.10.034 [Crossref] [ Google Scholar]
- Enomoto Y, Li P, Jenkins LM, Anastasakis D, Lyons GC, Hafner M. Cytokine-enhanced cytolytic activity of exosomes from NK cells. Cancer Gene Ther 2022; 29(6):734-49. doi: 10.1038/s41417-021-00352-2 [Crossref] [ Google Scholar]
- Jong AY, Wu CH, Li J, Sun J, Fabbri M, Wayne AS. Large-scale isolation and cytotoxicity of extracellular vesicles derived from activated human natural killer cells. J Extracell Vesicles 2017; 6(1):1294368. doi: 10.1080/20013078.2017.1294368 [Crossref] [ Google Scholar]
- McCune A, Kornbluth J. NK33-derived extracellular vesicles penetrate and selectively kill treatment-resistant tumor cells. Cancers (Basel) 2023; 16(1):90. doi: 10.3390/cancers16010090 [Crossref] [ Google Scholar]
- Kim HY, Min HK, Song HW, Yoo A, Lee S, Kim KP. Delivery of human natural killer cell-derived exosomes for liver cancer therapy: an in vivo study in subcutaneous and orthotopic animal models. Drug Deliv 2022; 29(1):2897-911. doi: 10.1080/10717544.2022.2118898 [Crossref] [ Google Scholar]
- Nathani A, Sun L, Khan I, Aare M, Bagde A, Li Y. Combined role of interleukin-15 stimulated natural killer cell-derived extracellular vesicles and carboplatin in osimertinib-resistant H1975 lung cancer cells with EGFR mutations. Pharmaceutics 2024; 16(1):83. doi: 10.3390/pharmaceutics16010083 [Crossref] [ Google Scholar]
- Cochran AM, Kornbluth J. Extracellular vesicles from the human natural killer cell line NK33 have broad and potent anti-tumor activity. Front Cell Dev Biol 2021; 9:698639. doi: 10.3389/fcell.2021.698639 [Crossref] [ Google Scholar]
- Matchett EC, Kornbluth J. Extracellular vesicles derived from immortalized human natural killer cell line NK33 as a novel therapeutic for multiple myeloma. Front Immunol 2023; 14:1265101. doi: 10.3389/fimmu.2023.1265101 [Crossref] [ Google Scholar]
- Lugini L, Cecchetti S, Huber V, Luciani F, Macchia G, Spadaro F. Immune surveillance properties of human NK cell-derived exosomes. J Immunol 2012; 189(6):2833-42. doi: 10.4049/jimmunol.1101988 [Crossref] [ Google Scholar]
- Di Pace AL, Tumino N, Besi F, Alicata C, Conti LA, Munari E. Characterization of human NK cell-derived exosomes: role of DNAM1 receptor in exosome-mediated cytotoxicity against tumor. Cancers (Basel) 2020; 12(3):661. doi: 10.3390/cancers12030661 [Crossref] [ Google Scholar]
- Han D, Wang K, Zhang T, Gao GC, Xu H. Natural killer cell-derived exosome-entrapped paclitaxel can enhance its anti-tumor effect. Eur Rev Med Pharmacol Sci 2020; 24(10):5703-13. doi: 10.26355/eurrev_202005_21362 [Crossref] [ Google Scholar]
- Kang YT, Niu Z, Hadlock T, Purcell E, Lo TW, Zeinali M. On-chip biogenesis of circulating NK cell-derived exosomes in non-small cell lung cancer exhibits antitumoral activity. Adv Sci 2021; 8(6):2003747. doi: 10.1002/advs.202003747 [Crossref] [ Google Scholar]
- Jiang Y, Jiang H, Wang K, Liu C, Man X, Fu Q. Hypoxia enhances the production and antitumor effect of exosomes derived from natural killer cells. Ann Transl Med 2021; 9(6):473. doi: 10.21037/atm-21-347 [Crossref] [ Google Scholar]
- Kaban K, Hinterleitner C, Zhou Y, Salva E, Kantarci AG, Salih HR. Therapeutic silencing of BCL-2 using NK cell-derived exosomes as a novel therapeutic approach in breast cancer. Cancers (Basel) 2021; 13(10):2397. doi: 10.3390/cancers13102397 [Crossref] [ Google Scholar]
- Zhang M, Shao W, Yang T, Liu H, Guo S, Zhao D. Conscription of immune cells by light-activatable silencing NK-derived exosome (LASNEO) for synergetic tumor eradication. Adv Sci (Weinh) 2022; 9(22):e2201135. doi: 10.1002/advs.202201135 [Crossref] [ Google Scholar]
- Parsonidis P, Mamagkaki A, Papasotiriou I. CTLs, NK cells and NK-derived EVs against breast cancer. Hum Immunol 2023; 84(5-7):320-6. doi: 10.1016/j.humimm.2023.03.001 [Crossref] [ Google Scholar]
- St-Denis-Bissonnette F, Cummings SE, Qiu S, Stalker A, Muradia G, Mehic J. A clinically relevant large-scale biomanufacturing workflow to produce natural killer cells and natural killer cell-derived extracellular vesicles for cancer immunotherapy. J Extracell Vesicles 2023; 12(12):e12387. doi: 10.1002/jev2.12387 [Crossref] [ Google Scholar]
- Di Pace AL, Pelosi A, Fiore PF, Tumino N, Besi F, Quatrini L. MicroRNA analysis of Natural Killer cell-derived exosomes: the microRNA let-7b-5p is enriched in exosomes and participates in their anti-tumor effects against pancreatic cancer cells. Oncoimmunology 2023; 12(1):2221081. doi: 10.1080/2162402x.2023.2221081 [Crossref] [ Google Scholar]
- St-Denis-Bissonnette F, Qiu S, Cummings SE, Kirkby M, Haile Y, Wassmer S. Evaluation of resazurin phenoxazine dye as a highly sensitive cell viability potency assay for natural killer cell-derived extracellular vesicle-based cancer biotherapeutics. J Extracell Biol 2024; 3(7):e166. doi: 10.1002/jex2.166 [Crossref] [ Google Scholar]
- Aarsund M, Nyman TA, Stensland ME, Wu Y, Inngjerdingen M. Isolation of a cytolytic subpopulation of extracellular vesicles derived from NK cells containing NKG7 and cytolytic proteins. Front Immunol 2022; 13:977353. doi: 10.3389/fimmu.2022.977353 [Crossref] [ Google Scholar]
- Zhu L, Gangadaran P, Kalimuthu S, Oh JM, Baek SH, Jeong SY. Novel alternatives to extracellular vesicle-based immunotherapy - exosome mimetics derived from natural killer cells. Artif Cells Nanomed Biotechnol 2018; 46(Suppl 3):S166-79. doi: 10.1080/21691401.2018.1489824 [Crossref] [ Google Scholar]
- Pitchaimani A, Nguyen TDT, Aryal S. Natural killer cell membrane infused biomimetic liposomes for targeted tumor therapy. Biomaterials 2018; 160:124-37. doi: 10.1016/j.biomaterials.2018.01.018 [Crossref] [ Google Scholar]
- Wang G, Hu W, Chen H, Shou X, Ye T, Xu Y. Cocktail strategy based on NK cell-derived exosomes and their biomimetic nanoparticles for dual tumor therapy. Cancers (Basel) 2019; 11(10):1560. doi: 10.3390/cancers11101560 [Crossref] [ Google Scholar]
- Goyal P, Goyal K, Vijaya Kumar SG, Singh A, Katare OP, Mishra DN. Liposomal drug delivery systems--clinical applications. Acta Pharm 2005; 55(1):1-25. [ Google Scholar]
- Singh R, Lillard JW Jr. Nanoparticle-based targeted drug delivery. Exp Mol Pathol 2009; 86(3):215-23. doi: 10.1016/j.yexmp.2008.12.004 [Crossref] [ Google Scholar]
- Walker S, Busatto S, Pham A, Tian M, Suh A, Carson K. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics 2019; 9(26):8001-17. doi: 10.7150/thno.37097 [Crossref] [ Google Scholar]
- Saari H, Lázaro-Ibáñez E, Viitala T, Vuorimaa-Laukkanen E, Siljander P, Yliperttula M. Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J Control Release 2015; 220(Pt B):727-37. doi: 10.1016/j.jconrel.2015.09.031 [Crossref] [ Google Scholar]
- Haney MJ, Zhao Y, Jin YS, Li SM, Bago JR, Klyachko NL. Macrophage-derived extracellular vesicles as drug delivery systems for triple negative breast cancer (TNBC) therapy. J Neuroimmune Pharmacol 2020; 15(3):487-500. doi: 10.1007/s11481-019-09884-9 [Crossref] [ Google Scholar]
- Hashemi ZS, Ghavami M, Kiaie SH, Mohammadi F, Shokrollahi Barough M, Khalili S. Novel delivery of sorafenib by natural killer cell-derived exosomes-enhanced apoptosis in triple-negative breast cancer. Nanomedicine (Lond) 2023; 18(5):437-53. doi: 10.2217/nnm-2022-0237 [Crossref] [ Google Scholar]
- Chen Y, Gao DY, Huang L. In vivo delivery of miRNAs for cancer therapy: challenges and strategies. Adv Drug Deliv Rev 2015; 81:128-41. doi: 10.1016/j.addr.2014.05.009 [Crossref] [ Google Scholar]
- Syromiatnikova V, Prokopeva A, Gomzikova M. Methods of the large-scale production of extracellular vesicles. Int J Mol Sci 2022; 23(18):10522. doi: 10.3390/ijms231810522 [Crossref] [ Google Scholar]
- Paganini C, Capasso Palmiero U, Pocsfalvi G, Touzet N, Bongiovanni A, Arosio P. Scalable production and isolation of extracellular vesicles: available sources and lessons from current industrial bioprocesses. Biotechnol J 2019; 14(10):e1800528. doi: 10.1002/biot.201800528 [Crossref] [ Google Scholar]
- Li YJ, Wu JY, Liu J, Xu W, Qiu X, Huang S. Artificial exosomes for translational nanomedicine. J Nanobiotechnology 2021; 19(1):242. doi: 10.1186/s12951-021-00986-2 [Crossref] [ Google Scholar]
- Zhao Z, Qu L, Shuang T, Wu S, Su Y, Lu F. Low-intensity ultrasound radiation increases exosome yield for efficient drug delivery. J Drug Deliv Sci Technol 2020; 57:101713. doi: 10.1016/j.jddst.2020.101713 [Crossref] [ Google Scholar]
- Hedlund M, Nagaeva O, Kargl D, Baranov V, Mincheva-Nilsson L. Thermal- and oxidative stress causes enhanced release of NKG2D ligand-bearing immunosuppressive exosomes in leukemia/lymphoma T and B cells. PLoS One 2011; 6(2):e16899. doi: 10.1371/journal.pone.0016899 [Crossref] [ Google Scholar]
- Ullah M, Liu DD, Rai S, Razavi M, Concepcion W, Thakor AS. Pulsed focused ultrasound enhances the therapeutic effect of mesenchymal stromal cell-derived extracellular vesicles in acute kidney injury. Stem Cell Res Ther 2020; 11(1):398. doi: 10.1186/s13287-020-01922-1 [Crossref] [ Google Scholar]
- Gao J, Wang S, Wang Z. High yield, scalable and remotely drug-loaded neutrophil-derived extracellular vesicles (EVs) for anti-inflammation therapy. Biomaterials 2017; 135:62-73. doi: 10.1016/j.biomaterials.2017.05.003 [Crossref] [ Google Scholar]
- Khosravi N, Pishavar E, Baradaran B, Oroojalian F, Mokhtarzadeh A. Stem cell membrane, stem cell-derived exosomes and hybrid stem cell camouflaged nanoparticles: a promising biomimetic nanoplatforms for cancer theranostics. J Control Release 2022; 348:706-22. doi: 10.1016/j.jconrel.2022.06.026 [Crossref] [ Google Scholar]