Biomed. adv. 2(1):3-9.
doi: 10.34172/bma.09
Review Article
Optimized protocol for esophagectomy to improve hemodynamic stability and minimize ICU stay duration: A methodological study with specific focus on intraoperative care
Amirhosein Hashemzadeh Investigation, Methodology, 1 
Shahriyar Hashemzadeh Conceptualization, Supervision, Visualization, 2 
Shadi Khodaei Methodology, Writing – review & editing, 1 
Arman Hashemzadeh Methodology, Writing – review & editing, 1 
Marjan Dehdilani Conceptualization, Investigation, Validation, Visualization, 3, * 
Author information:
1Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran
2Tuberculosis and Lung Disease Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
3Department of Anesthesiology, School of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Summary
Patients undergoing esophagectomy face distinct challenges in maintaining hemodynamic stability and require rigorous monitoring in the intensive care unit (ICU); thus, establishing a newly designed intraoperative care guideline could reduce complications and enhance hemodynamic stability during the first 24 hours in the ICU. This study aims to develop a standardized intraoperative care guideline for patients undergoing esophagectomy to enhance postoperative care, improve hemodynamic stability, and reduce the length of ICU stay. This methodological study, carried out in 2024 at Imam Reza hospital under Tabriz University of Medical Sciences in Iran, aimed to develop and apply intraoperative care guidelines for patients undergoing esophagectomy. A thorough literature review was performed, incorporating high-quality research on intraoperative care, focusing on enhancing hemodynamic stability and shortening ICU stays. The Delphi method was employed to achieve expert consensus on the finalized guidelines. In this study, we extracted intraoperative factors that contribute to hemodynamic stability and reduced hospital length of stay in patients undergoing esophagectomy from high-quality studies and presented them in the form of a guideline. The tailored intraoperative care guideline markedly enhanced hemodynamic stability during the early postoperative period after esophagectomy. This approach has the potential to lower complication risks and promote patient recovery. Additional research is required to evaluate its long-term outcomes and cost-effectiveness.
Keywords: Intraoperative, Caring guideline, Hemodynamic stability, Esophagectomy
Copyright and License Information
© 2025 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
This work was supported by Deputy for Research of Tabriz University of Medical Sciences.
Introduction
Esophagectomy is a complex and high-risk surgical procedure frequently performed to treat esophageal cancer, benign esophageal diseases, and various other conditions.1 Despite significant advances in surgical techniques and perioperative care, esophagectomy remains associated with high morbidity and mortality rates, particularly due to postoperative complications such as anastomotic leakage, infection, respiratory failure, and hemodynamic instability.2 These complications often necessitate prolonged intensive care unit (ICU) stays, further complicating patient recovery and increasing healthcare costs.3
The maintenance of hemodynamic stability throughout the perioperative period is critical to achieving successful outcomes after esophagectomy.4 Hemodynamic instability during surgery and the early postoperative period can contribute to various adverse outcomes, including organ dysfunction, shock, and delayed recovery. Furthermore, prolonged ICU stays are linked to increased risks of hospital-acquired infections, muscle weakness, and thromboembolic events, which can significantly hinder patient recovery.5
Effective perioperative care strategies aimed at optimizing hemodynamic stability, minimizing surgical stress, and promoting early recovery are essential in reducing complications and improving patient outcomes.6 This includes preoperative optimization, intraoperative hemodynamic management, and postoperative interventions focused on pain control, respiratory support, and early mobilization.7
This study aims to provide a comprehensive guideline for the intraoperative management of patients undergoing esophagectomy, with a specific focus on strategies that enhance hemodynamic stability and reduce the length of ICU stay. Through evidence-based practices and multidisciplinary care, it is possible to significantly improve the prognosis of these patients, minimize complications, and accelerate recovery.
Material and Methods
Study design
This methodological study, conducted at Imam Reza Hospital under the affiliation of Tabriz University of Medical Sciences, targeted patients undergoing esophagectomy. The study aimed to design and implement intraoperative care strategies to improve hemodynamic stability and reduce ICU stay durations.
Search strategy
A comprehensive search from electronic databases including MEDLINE, PubMed, Scopus, Web of Science, and Google Scholar was initially performed covering the period from 1995 to 2023. This search focused on high-quality publications, including randomized controlled trials, cohort studies, systematic reviews, and meta-analyses. Studies that addressed intraoperative care before, during, and after esophagectomy, especially those that discussed interventions leading to improved hemodynamic status and reduced hospital stays, were included in the review. Each subject was considered based on at least three relevant studies, and similar topics were merged, with duplicate articles removed. The items were read multiple times for clarity, grammar, and understanding.
Evidence quality and recommendations
The authors evaluated the quality of evidence and strength of recommendations based on the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) system. Evidence quality was ranked into three categories: high, moderate, and low. Only studies with high-quality evidence were included in the review. Recommendations were also assessed at three levels: strong, moderate, or weak. Strong recommendations were made when the benefits of an intervention outweighed its harms, while moderate or weak recommendations were based on either low-quality evidence or when the benefits and harms were equally balanced. Only studies with strong recommendations were included.8
Delphi method for finalizing the guidelines
The Delphi method was used to finalize the guidelines and ensure consensus among experts. The Delphi process involved gathering insights from a panel of specialists to reach an agreement on complex issues related to perioperative care for esophagectomy patients. A team consisting of a thoracic surgeon, an anesthesiologist, and an epidemiologist was formed. The panel reviewed all items extracted from the literature and documented evidence-based recommendations. The main issue requiring expert decision-making was identified, and a focused question was formulated. The topic was shared with the experts for approval, rejection, or modification. An initial questionnaire was developed based on the identified needs of the expert panel. Each question addressed specific aspects of intraoperative care for esophagectomy patients. The questionnaire was distributed to the Delphi panel members, who were selected from a specialized database of anesthesiologists, thoracic surgeons, and epidemiologists. They completed the questionnaire based on their experience and expertise. Responses were analyzed to identify areas of consensus and disagreement. Based on the initial analysis, the second version of the questionnaire was developed with clearer and more refined questions. This process may have been repeated several times to refine the questions. The panel continued to analyze and revise the questions until a consensus was reached. Decisions were made regarding points of agreement, and any issues requiring further clarification were addressed. The panel carried out a final review of the guidelines to verify their accuracy, thoroughness, and consistency with the evidence. Following this, a detailed report was compiled, summarizing the findings and the group’s decisions.
Results
Intraoperative care
The following outlines the key principles of intraoperative Care. The following outlines the key principles of intraoperative Care (Figure 1).
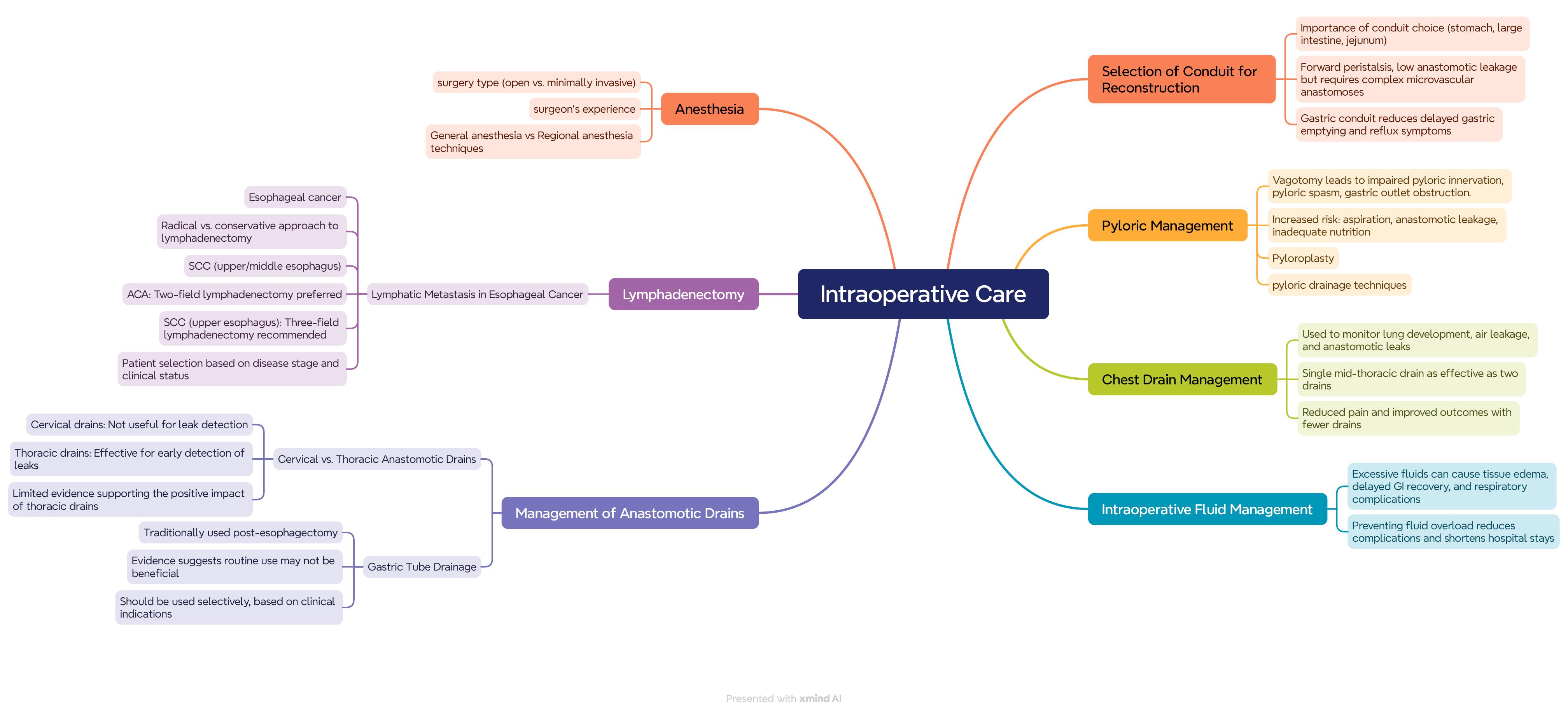
Figure 1.
Intraoperative care guideline
.
Intraoperative care guideline
Selection of channel type
Selecting the optimal reconstruction channel after esophageal resection, particularly among gastric, colonic, and jejunal options, is critically important. Although jejunal channels have forward peristaltic contractions and a lower leakage rate, this method requires complex microvascular anastomoses to reach the chest or neck region.9 Since there are no randomized controlled trials (RCTs) comparing these channel options and controlled observational studies are rare, available data primarily consist of case series, which have contradictory findings on long-term quality of life differences and functional outcomes.10 A systematic review of two RCTs and five cohort studies suggest that reconstruction with a gastric channel, compared to a tubular gastric channel, reduces delayed gastric emptying, presents fewer reflux symptoms, and offers better quality of life. Overall, acceptable options for esophageal reconstruction include the stomach, colon, and jejunum, with decisions based on the advantages and limitations of each option.11
Pylorus management
Optimal pylorus management during esophageal surgery remains unclear. Vagotomy, commonly performed with esophageal surgery, can lead to loss of pyloric innervation, potentially causing pyloric spasm and gastric outlet obstruction. This can increase the risk of aspiration, anastomotic leakage, and inadequate feeding.12 Although many surgeons routinely perform pyloroplasty for pyloric drainage, potential drawbacks such as biliary reflux and the risk of leakage at the suture site exist.13 Evidence on the impact of different pyloric drainage techniques, including pyloroplasty, is limited, thus no specific recommendations can be made.14,15
Lymphadenectomy
Esophageal cancer is associated with a high rate of lymph node metastasis, which varies depending on the type of cancer. The dissection of lymph node stations is often debated, with some surgeons opting for a radical approach while others take a more conservative approach.16 For squamous cell carcinoma (SCC) in the upper and middle regions, a three-field lymphadenectomy (including abdominal and mediastinal lymph nodes) is recommended.17 Evidence suggests that three-field lymphadenectomy improves survival for SCC in the upper and middle esophagus, though this benefit is not observed in cases with five or more positive lymph nodes. Consequently, a two-field lymphadenectomy is recommended for adenocarcinoma, and a three-field lymphadenectomy for SCC in the upper esophagus, with precise patient selection based on disease stage and general condition.18
Management of anastomotic drains
Anastomotic drains in esophageal surgery should be divided into two categories: cervical and chest drains. Cervical drains typically do not provide clinical information related to leaks and are not beneficial in this regard.19 Chest drains are valuable for the timely diagnosis and management of leaks, although the available evidence supporting their efficacy remains limited. Therefore, cervical anastomotic drains are not recommended, while chest drains show limited evidence of a positive impact.20
Gastric tube decompression
The use of gastric tubes after esophageal surgery is traditionally considered to reduce respiratory complications and issues related to anastomotic leaks.21 However, evidence suggests that routine use of these tubes may not lead to optimal outcomes, and their use should be selective. Research suggests that removing the gastric tube early, on the second postoperative day, may help reduce respiratory complications. However, further studies are needed to confirm these findings.22,23
Management of chest drains
Fluid drainage from the chest is a routine practice in most centers following esophageal surgery, facilitating full lung expansion and enabling the monitoring of air, chyle, and anastomotic leaks. Although various methods exist for managing drains,23,24 it seems that using a single drain in the mid-chest is as effective as using two and may reduce pain. Therefore, drain use should be minimized, and drains can be removed if no leakage is present.25
Use of nutritional tubes
Postoperative feeding after esophageal surgery may be enteral or parenteral, with most data favoring enteral feeding.26 This type of feeding reduces surgical stress and complications, including anastomotic leakage. Based on available evidence, early enteral feeding should begin 3-6 days post-surgery, and the target nutritional rate should be established.27,28
Intraoperative fluid management
Intraoperative fluid management directly impacts postoperative complications.29 Excessive fluids may lead to tissue edema, delayed return to normal gastrointestinal function, and increased respiratory complications.30 Therefore, preventing fluid overload can reduce major complications and shorten hospital stay.31
Anesthesia techniques
The choice of anesthesia in esophageal surgeries depends on several factors, including the type of surgery (open or minimally invasive), the patient’s clinical status, and the surgical team’s experience. In esophagectomy surgeries, general anesthesia is typically used, providing complete control over respiration and patient relaxation.32,33 The use of adjuncts like regional anesthesia (if necessary) may help reduce the need for general anesthesia drugs and improve postoperative outcomes.34,35
Discussion
Preoperative and intraoperative interventions and thorough patient preparation are essential for enhancing surgical outcomes and minimizing postoperative complications. These steps help identify and manage surgical risks, allowing patients to prepare for the surgical experience adequately. The initial step is a comprehensive clinical assessment, which includes taking a medical history, monitoring vital signs, and conducting a physical examination.36 This evaluation enables the physician to identify potential risk factors and take the necessary actions. Additionally, diagnostic tests such as blood tests and imaging studies help determine the patient’s overall health status, potentially influencing the type of surgery and the need for further preparations.37
Medication management before surgery is also of high importance. Physicians should compile a list of the patient’s medications and decide which ones should continue and which should be stopped.38 Special attention should be given to anticoagulant drugs and medications related to chronic conditions, such as diabetes and hypertension, to minimize surgical risks. Furthermore, informing the patient about medication timing, consumption, and necessary adjustments before surgery is critical.39
Nutrition and hydration are other vital aspects of patient preparation. Physicians must guide patients on dietary and fluid restrictions before surgery, particularly in general surgeries and anesthesia, as these restrictions significantly affect patient health and can reduce the risk of complications like aspiration. Additionally, informing patients about the upcoming procedure and what to expect on the surgery day can help reduce anxiety and alleviate concerns.37,40
Furthermore, the psychological preparation of the patient should not be overlooked. Effective communication with the patient and providing sufficient information about the surgical process, potential complications, and expected outcomes can help build trust and reduce anxiety. Preoperative counseling sessions can assist the patient in feeling more confident about the process and better equipped to face it.41
Finally, establishing a multidisciplinary team that includes physicians, nurses, and other specialists for comprehensive and coordinated patient preparation is essential. This team can adopt the best practices for each patient through close collaboration and information exchange, ultimately improving surgical quality and outcomes.42 All these preoperative measures not only enhance patient safety but also positively impact the overall patient experience during and after surgery. Given these factors, it is clear that proper preoperative preparation is a multifaceted and complex process that requires attention to detail and collaboration among the healthcare team members.5
Intraoperative actions and recommendations in guidelines are crucial for enhancing patient safety and improving the quality of the surgical procedure21. This section emphasizes the adherence to precise surgical protocols and the use of standard techniques during the procedure as key factors in reducing complications and improving surgical outcomes. One of the most important aspects in this regard is the meticulous implementation of safety checklists at different stages of surgery. These checklists should be reviewed continuously, not only before surgery but also throughout the procedure. For instance, confirming the patient’s identity, the type of surgery, and checking the surgical instruments at each stage can prevent human errors and ensure patient safety.33
Additionally, minimally invasive surgical techniques and advanced methods, such as robotic surgery, are recognized as effective approaches for reducing complications and shortening recovery time.17 These techniques allow surgeons to perform the procedure with greater precision, causing less damage to surrounding tissues. Furthermore, they can potentially reduce postoperative bleeding and pain. Given this, guidelines recommend that surgeons stay up-to-date and incorporate the latest scientific advancements in their surgical practices.43
Proper management of medications and anesthesia during surgery is another critical component of these guidelines. Continuous monitoring of the patient’s status and their response to anesthetic drugs can help detect potential problems and complications early. This is particularly important for patients with specific medical conditions.44 Close collaboration between the surgical and anesthesia teams is essential for improving the quality of the surgery and ensuring patient safety.2
Moreover, maintaining hygiene and infection control during surgery is another key measure outlined in these guidelines.45 Correct sterilization techniques, disinfecting surgical tools, and following hygiene protocols by the surgical team can significantly reduce the risk of postoperative infections. Continuous training for surgical staff on hygiene and safety can improve service quality and increase patient trust.46
Attention to patients’ psychological and emotional status during surgery is also important. Creating a calm and supportive environment can help reduce patient anxiety and positively affect surgical outcomes.47 Effective communication with patients, explaining the surgical process, and addressing their concerns are measures that can be impactful in this area.48
Lastly, the continuous evaluation and assessment of the surgical team’s performance and the outcomes of surgeries contribute to identifying strengths and weaknesses in the surgical process.49 This evaluation can involve recording and analyzing data on complications, recovery times, and patient satisfaction. With this information, the surgical team can continuously improve the quality of their services and effectively work on reducing complications and enhancing surgical outcomes. Adherence to intraoperative guidelines directly influences surgical safety and quality, leading to an overall improvement in patient experience and increased trust in healthcare services.50
In addition to intraoperative care, postoperative actions are a vital phase in the patient’s treatment process, significantly impacting surgical outcomes and reducing complications.51 These actions are especially critical in the early hours after surgery when the patient is under the effects of anesthesia and physiological changes. The first and most important action is continuous monitoring of the patient’s vital signs, including heart rate, blood pressure, and oxygen saturation.6 These assessments help quickly identify and manage any adverse changes in the patient’s condition. During this phase, attention to vital signs and necessary evaluations can reduce the risk of serious complications such as shock or infection.51
Pain management after surgery is another key element of postoperative care. Implementing appropriate pain relief protocols, especially for patients undergoing major and invasive surgeries, directly impacts their recovery quality.52 Pain medications should be prescribed accurately and timely, and medical staff should receive proper training on recognizing and managing patient pain. Furthermore, addressing the patient’s psychological and emotional well-being is crucial, as comfort and relaxation can aid in a quicker and more effective recovery.53
Additionally, proper wound care is essential post-surgery. Regular wound inspection, cleanliness, and identifying signs of infection should be performed. Educating the patient on how to care for the wound, recognize infection signs, and know when to seek medical attention can enhance surgical outcomes and minimize complications. Additionally, using appropriate dressings and sterile techniques for dressing changes will help reduce the risk of infection.54
Postoperative nutrition is another important aspect of care. Based on the type of surgery and the patient’s condition, planning for proper nutrition can accelerate recovery and reduce complications like malnutrition or infection. Collaborating with nutrition specialists and providing necessary guidance to the patient and their family can play a significant role in improving the patient’s quality of life.55
Lastly, planning for follow-up and return to daily activities is essential in the postoperative period. This includes determining the appropriate time for resuming normal activities, exercise, and work. Providing the necessary counseling in this regard can help patients continue their recovery process effectively and prevent complications from inactivity or premature activity. Overall, postoperative actions directly affect the patient’s recovery process and should be carried out comprehensively and systematically to achieve the best possible outcomes.39
Conclusion
Appropriate measures before, during, and after surgery are recognized as key elements in improving treatment outcomes and reducing complications. These measures include thorough preoperative patient assessment, continuous monitoring during surgery, effective pain management, wound care, as well as attention to nutrition, and postoperative follow-up. Implementing standard protocols and comprehensive planning can enhance patients’ quality of life and increase their satisfaction levels. Ultimately, focusing on the details of these actions and fostering collaboration among medical and nursing teams is crucial in achieving optimal outcomes.
Competing Interests
No conflict of interest in this work.
Ethical Approval
Nor applicable.
Acknowledgements
The research protocol was approved and supported by the Student Research Committee, Tabriz University of Medical Sciences (grant number: 74405).
References
- Tagkalos E, van der Sluis PC, Berlth F, Poplawski A, Hadzijusufovic E, Lang H. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy versus minimally invasive esophagectomy for resectable esophageal adenocarcinoma, a randomized controlled trial (ROBOT-2 trial). BMC Cancer 2021; 21(1):1060. doi: 10.1186/s12885-021-08780-x [Crossref] [ Google Scholar]
- Wang H, Tang H, Fang Y, Tan L, Yin J, Shen Y. Morbidity and mortality of patients who underwent minimally invasive esophagectomy after neoadjuvant chemoradiotherapy vs neoadjuvant chemotherapy for locally advanced esophageal squamous cell carcinoma: a randomized clinical trial. JAMA Surg 2021; 156(5):444-51. doi: 10.1001/jamasurg.2021.0133 [Crossref] [ Google Scholar]
- Khan SH, Perkins AJ, Jawaid S, Wang S, Lindroth H, Schmitt RE. Serum proteomic analysis in esophagectomy patients with postoperative delirium: a case-control study. Heart Lung 2024; 63:35-41. doi: 10.1016/j.hrtlng.2023.09.009 [Crossref] [ Google Scholar]
- Mukai A, Suehiro K, Watanabe R, Juri T, Hayashi Y, Tanaka K. Impact of intraoperative goal-directed fluid therapy on major morbidity and mortality after transthoracic oesophagectomy: a multicentre, randomised controlled trial. Br J Anaesth 2020; 125(6):953-61. doi: 10.1016/j.bja.2020.08.060 [Crossref] [ Google Scholar]
- Yang C, Shi Y, Zhang M, Yang Y, Xie Y. Impact of staged goal-directed fluid therapy on postoperative pulmonary complications in patients undergoing McKeown esophagectomy: a randomized controlled trial. BMC Anesthesiol 2024; 24(1):330. doi: 10.1186/s12871-024-02719-y [Crossref] [ Google Scholar]
- Hu J, Zhu M, Gao Z, Zhao S, Feng X, Chen J. Dexmedetomidine for prevention of postoperative delirium in older adults undergoing oesophagectomy with total intravenous anaesthesia: a double-blind, randomised clinical trial. Eur J Anaesthesiol 2021; 38(Suppl 1):S9-17. doi: 10.1097/eja.0000000000001382 [Crossref] [ Google Scholar]
- Xu H, Shu SH, Wang D, Chai XQ, Xie YH, Zhou WD. Goal-directed fluid restriction using stroke volume variation and cardiac index during one-lung ventilation: a randomized controlled trial. J Thorac Dis 2017; 9(9):2992-3004. doi: 10.21037/jtd.2017.08.98 [Crossref] [ Google Scholar]
- Piggott T, Morgan RL, Cuello-Garcia CA, Santesso N, Mustafa RA, Meerpohl JJ. Grading of Recommendations Assessment, Development, and Evaluations (GRADE) notes: extremely serious, GRADE’s terminology for rating down by three levels. J Clin Epidemiol 2020; 120:116-20. doi: 10.1016/j.jclinepi.2019.11.019 [Crossref] [ Google Scholar]
- Flanagan JC, Batz R, Saboo SS, Nordeck SM, Abbara S, Kernstine K. Esophagectomy and gastric pull-through procedures: surgical techniques, imaging features, and potential complications. Radiographics 2016; 36(1):107-21. doi: 10.1148/rg.2016150126 [Crossref] [ Google Scholar]
- Kitadani J, Ojima T, Hayata K, Goda T, Takeuchi A, Katsuda M. Nutritional benefit of remnant gastric preservation in patients with esophageal cancer undergoing radical esophagectomy and ileo-colon interposition. BMC Surg 2022; 22(1):255. doi: 10.1186/s12893-022-01704-x [Crossref] [ Google Scholar]
- Gentile D, Riva P, Da Roit A, Basato S, Marano S, Castoro C. Gastric tube cancer after esophagectomy for cancer: a systematic review. Dis Esophagus 2019; 32(8):doz049. doi: 10.1093/dote/doz049 [Crossref] [ Google Scholar]
- Kykalos S, Ntikoudi E. Management of pylorus during esophagectomy. To drain or not to drain? Chirurgia (Bucur) 2018; 113(1):162-3. doi: 10.21614/chirurgia.113.1.162 [Crossref] [ Google Scholar]
- Benz C, Martella J, Hamwi B, Okereke I. Factors resulting in postoperative dysphagia following esophagectomy: a narrative review. J Thorac Dis 2021; 13(7):4511-8. doi: 10.21037/jtd-21-724 [Crossref] [ Google Scholar]
- Ferreira Junior EG, Costa PA, Freire Golveia Silveira LM, Pertile Salvioni NC, Loureiro BM, Lodi Peres SL. Transhiatal esophagectomy with gastric pull-up, pyloric exclusion and Roux-en-Y gastroenterostomy for the management of esophageal caustic injury. Int J Surg Case Rep 2019; 56:66-9. doi: 10.1016/j.ijscr.2019.02.006 [Crossref] [ Google Scholar]
- Honig SE, Lundgren MP, Kowalski TE, Lavu H, Yeo CJ. Advanced endoscopic rescue of a complication (duodenojejunostomy leak) after a pylorus-preserving pancreaticoduodenectomy in a post-esophagectomy patient with pancreatic adenocarcinoma: a case report and review of the literature. J Pancreat Cancer 2020; 6(1):5-11. doi: 10.1089/pancan.2019.0016 [Crossref] [ Google Scholar]
- Nadkarni S, Jiwnani S, Reddy VS, Niyogi D, Tiwari VK, Karimundackal G, et al. Robotic esophagectomy and 3-field lymphadenectomy with intraoperative nerve monitoring. Multimed Man Cardiothorac Surg 2022;2022. 10.1510/mmcts-2022-067.
- Low DE, Allum W, De Manzoni G, Ferri L, Immanuel A, Kuppusamy M. Guidelines for perioperative care in esophagectomy: enhanced recovery after surgery (ERAS®) society recommendations. World J Surg 2019; 43(2):299-330. doi: 10.1007/s00268-018-4786-4 [Crossref] [ Google Scholar]
- Kingma BF, Ruurda JP, van Hillegersberg R. An editorial on lymphadenectomy in esophagectomy for cancer. Ann Surg Oncol 2022; 29(8):4676-8. doi: 10.1245/s10434-022-11736-5 [Crossref] [ Google Scholar]
- Latorre-Rodríguez AR, Huang J, Schaheen L, Smith MA, Hashimi S, Bremner RM. Diagnosis and management of anastomotic leaks after Ivor Lewis esophagectomy: a single-center experience. Langenbecks Arch Surg 2023; 408(1):397. doi: 10.1007/s00423-023-03121-x [Crossref] [ Google Scholar]
- Barbaro A, Eldredge TA, Shenfine J. Diagnosing anastomotic leak post-esophagectomy: a systematic review. Dis Esophagus 2021; 34(2):doaa076. doi: 10.1093/dote/doaa076 [Crossref] [ Google Scholar]
- Watkins AA, Kent MS, Wilson JL. Surgical adjuncts during esophagectomy. Thorac Surg Clin 2020; 30(3):315-20. doi: 10.1016/j.thorsurg.2020.04.009 [Crossref] [ Google Scholar]
- Weijs TJ, Kumagai K, Berkelmans GH, Nieuwenhuijzen GA, Nilsson M, Luyer MD. Nasogastric decompression following esophagectomy: a systematic literature review and meta-analysis. Dis Esophagus 2017; 30(3):1-8. doi: 10.1111/dote.12530 [Crossref] [ Google Scholar]
- Liu B, Wang W, Liang T. Clinical observation of modified gastric tube in middle and lower thoracic esophageal carcinoma surgery. J Cardiothorac Surg 2019; 14(1):146. doi: 10.1186/s13019-019-0967-y [Crossref] [ Google Scholar]
- Fujita T, Daiko H. Efficacy and predictor of octreotide treatment for postoperative chylothorax after thoracic esophagectomy. World J Surg 2014; 38(8):2039-45. doi: 10.1007/s00268-014-2501-7 [Crossref] [ Google Scholar]
- Hamada J, Konishi H, Shiozaki A, Fujiwara H, Shoda K, Kosuga T. Management of pleural effusion after mediastinoscopic radical esophagectomy. Anticancer Res 2018; 38(12):6919-25. doi: 10.21873/anticanres.13069 [Crossref] [ Google Scholar]
- Davies SJ, Wheelwright S. The impact of jejunostomy feeding on nutritional outcomes after oesophagectomy. J Hum Nutr Diet 2024; 37(1):126-36. doi: 10.1111/jhn.13235 [Crossref] [ Google Scholar]
- Ishikawa Y, Nishikawa K, Fukushima N, Takahashi K, Hasegawa Y, Yuda M. Assessment of button-type jejunostomy for nutritional management after esophagectomy in 201 cases. Int J Clin Oncol 2021; 26(12):2224-8. doi: 10.1007/s10147-021-02022-7 [Crossref] [ Google Scholar]
- Schattner M. Enteral nutritional support of the patient with cancer: route and role. J Clin Gastroenterol 2003; 36(4):297-302. doi: 10.1097/00004836-200304000-00004 [Crossref] [ Google Scholar]
- Ashok A, Niyogi D, Ranganathan P, Tandon S, Bhaskar M, Karimundackal G. The enhanced recovery after surgery (ERAS) protocol to promote recovery following esophageal cancer resection. Surg Today 2020; 50(4):323-34. doi: 10.1007/s00595-020-01956-1 [Crossref] [ Google Scholar]
- Takahashi M, Toyama H, Takahashi K, Kaiho Y, Ejima Y, Yamauchi M. Impact of intraoperative fluid management on postoperative complications in patients undergoing minimally invasive esophagectomy for esophageal cancer: a retrospective single-center study. BMC Anesthesiol 2024; 24(1):29. doi: 10.1186/s12871-024-02410-2 [Crossref] [ Google Scholar]
- Neal JM, Wilcox RT, Allen HW, Low DE. Near-total esophagectomy: the influence of standardized multimodal management and intraoperative fluid restriction. Reg Anesth Pain Med 2003; 28(4):328-34. doi: 10.1016/s1098-7339(03)00197-4 [Crossref] [ Google Scholar]
- Carney A, Dickinson M. Anesthesia for esophagectomy. Anesthesiol Clin 2015; 33(1):143-63. doi: 10.1016/j.anclin.2014.11.009 [Crossref] [ Google Scholar]
- Zhang YT, Chen Y, Shang KX, Yu H, Li XF, Yu H. Effect of volatile anesthesia versus intravenous anesthesia on postoperative pulmonary complications in patients undergoing minimally invasive esophagectomy: a randomized clinical trial. Anesth Analg 2024; 139(3):571-80. doi: 10.1213/ane.0000000000006814 [Crossref] [ Google Scholar]
- Lerut T, Wiesel O. History of esophagectomy for cancer of the esophagus and the gastroesophageal junction. Ann Transl Med 2021; 9(10):897. doi: 10.21037/atm-21-676 [Crossref] [ Google Scholar]
- Durkin C, Schisler T, Lohser J. Current trends in anesthesia for esophagectomy. Curr Opin Anaesthesiol 2017; 30(1):30-5. doi: 10.1097/aco.0000000000000409 [Crossref] [ Google Scholar]
- Wang P, Lei M, Chen Y, He H, Lin J, Lin H. Prognostic factors and outcomes in elderly esophagectomy patients with esophageal cancer. Ann Surg Oncol 2024; 31(3):1553-61. doi: 10.1245/s10434-023-14634-6 [Crossref] [ Google Scholar]
- Veelo DP, Geerts BF. Anaesthesia during oesophagectomy. J Thorac Dis 2017; 9(Suppl 8):S705-12. doi: 10.21037/jtd.2017.03.153 [Crossref] [ Google Scholar]
- Or C, Das R, Despotovic I, Alibhai AY, Moult E, Waheed NK. Combined multimodal analysis of peripheral retinal and macular circulation in diabetic retinopathy (COPRA Study). Ophthalmol Retina 2019; 3(7):580-8. doi: 10.1016/j.oret.2019.03.001 [Crossref] [ Google Scholar]
- Bolger JC, Loughney L, Tully R, Cunningham M, Keogh S, McCaffrey N. Perioperative prehabilitation and rehabilitation in esophagogastric malignancies: a systematic review. Dis Esophagus 2019; 32(9):doz058. doi: 10.1093/dote/doz058 [Crossref] [ Google Scholar]
- Mao Y, Sun X, Si L, Chen L, Liu X, Zhang Z. Perioperative dexmedetomidine fails to improve postoperative analgesic consumption and postoperative recovery in patients undergoing lateral thoracotomy for thoracic esophageal cancer: a randomized, double-blind, placebo-controlled trial. Pain Res Manag 2020; 2020:4145893. doi: 10.1155/2020/4145893 [Crossref] [ Google Scholar]
- Sivakumar J, Sivakumar H, Read M, Sinclair RC, Snowden CP, Hii MW. The role of cardiopulmonary exercise testing as a risk assessment tool in patients undergoing oesophagectomy: a systematic review and meta-analysis. Ann Surg Oncol 2020; 27(10):3783-96. doi: 10.1245/s10434-020-08638-9 [Crossref] [ Google Scholar]
- Shargall Y, Wiercioch W, Brunelli A, Murthy S, Hofstetter W, Lin J, et al. Joint 2022 European Society of Thoracic Surgeons and The American Association for Thoracic Surgery guidelines for the prevention of cancer-associated venous thromboembolism in thoracic surgery. J Thorac Cardiovasc Surg 2023;165(3):794-824.e6. 10.1016/j.jtcvs.2022.05.041.
- Dadgar N, Edlukudige Keshava V, Raj MS, Wagner PL. The influence of the microbiome on immunotherapy for gastroesophageal cancer. Cancers (Basel) 2023; 15(18):4426. doi: 10.3390/cancers15184426 [Crossref] [ Google Scholar]
- Shemmeri E, Wee JO. Minimally invasive modified McKeown esophagectomy. Surg Oncol Clin N Am 2024; 33(3):509-17. doi: 10.1016/j.soc.2023.12.020 [Crossref] [ Google Scholar]
- Bower MR, Martin RC, 2nd 2nd. Nutritional management during neoadjuvant therapy for esophageal cancer. J Surg Oncol 2009; 100(1):82-7. doi: 10.1002/jso.21289 [Crossref] [ Google Scholar]
- Na KJ, Kang CH, Kim YR, Kang MJ, Song EH, Jang EJ, et al. Comparison of clinical outcomes and postoperative nutritional status between early and late oral feeding after esophagectomy: an open labeled randomized controlled trial. Ann Surg. 2024. 10.1097/sla.0000000000006441.
- Tsang JS, Tong DK, Lam KO, Law BTT, Wong IY, Chan DK. Appropriate timing for surgery after neoadjuvant chemoradiation for esophageal cancer. Dis Esophagus 2017; 30(9):1-8. doi: 10.1093/dote/dox062 [Crossref] [ Google Scholar]
- Pinto E, Cavallin F, Scarpa M. Psychological support of esophageal cancer patient?. J Thorac Dis 2019; 11(Suppl 5):S654-62. doi: 10.21037/jtd.2019.02.34 [Crossref] [ Google Scholar]
- Vetter D, Gutschow CA. Strategies to prevent anastomotic leakage after esophagectomy and gastric conduit reconstruction. Langenbecks Arch Surg 2020; 405(8):1069-77. doi: 10.1007/s00423-020-01926-8 [Crossref] [ Google Scholar]
- Okada N, Fujita T, Kanamori J, Sato A, Kurita D, Horikiri Y. Efficacy of prewarming prophylaxis method for intraoperative hypothermia during thoracoscopic esophagectomy. Esophagus 2020; 17(4):385-91. doi: 10.1007/s10388-020-00743-8 [Crossref] [ Google Scholar]
- An KR, Seijas V, Xu MS, Grüßer L, Humar S, Moreno AA. Does prehabilitation before esophagectomy improve postoperative outcomes? A systematic review and meta-analysis. Dis Esophagus 2024; 37(3):doad066. doi: 10.1093/dote/doad066 [Crossref] [ Google Scholar]
- Ağagündüz D, Cocozza E, Cemali Ö, Bayazıt AD, Nanì MF, Cerqua I. Understanding the role of the gut microbiome in gastrointestinal cancer: A review. Front Pharmacol 2023; 14:1130562. doi: 10.3389/fphar.2023.1130562 [Crossref] [ Google Scholar]
- Rosner AK, van der Sluis PC, Meyer L, Wittenmeier E, Engelhard K, Grimminger PP. Pain management after robot-assisted minimally invasive esophagectomy. Heliyon 2023; 9(3):e13842. doi: 10.1016/j.heliyon.2023.e13842 [Crossref] [ Google Scholar]
- Steenhagen E. Preoperative nutritional optimization of esophageal cancer patients. J Thorac Dis 2019; 11(Suppl 5):S645-53. doi: 10.21037/jtd.2018.11.33 [Crossref] [ Google Scholar]
- Cao J, Xu H, Li W, Guo Z, Lin Y, Shi Y. Nutritional assessment and risk factors associated to malnutrition in patients with esophageal cancer. Curr Probl Cancer 2021; 45(1):100638. doi: 10.1016/j.currproblcancer.2020.100638 [Crossref] [ Google Scholar]