Biomed. adv. 1(1):34-41.
doi: 10.34172/bma.06
Original Article
Impact of Allopurinol on early and one-year outcomes of patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention: A randomized controlled trial
Nilgoon Daviran Conceptualization, Writing – review & editing, 1 
Amirhosein Ghafouri-Asbagh Formal analysis, Methodology, 1 
Hooman Nateghian Formal analysis, Methodology, Writing – review & editing, 2, * 
Ahmad Separham Conceptualization, Funding acquisition, Investigation, Validation, 1 
Bahram Sohrabi Formal analysis, Visualization, 1 
Naser Aslanabadi Investigation, Supervision, Validation, 1 
Mehrdad Raadi Investigation, Methodology, 1 
Author information:
1Cardiovascular Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2Research Center for Evidence‑Based Medicine, Iranian EBM Centre: A Joanna Briggs Institute Affiliated Group, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Summary
Introduction:
Due to the potential benefits of allopurinol in ischemic reperfusion injury, this randomized control trial was performed to evaluate the pretreatment allopurinol effect on major adverse cardiovascular events (MACE) in patients undergoing primary percutaneous coronary intervention (pPCI).
Methods:
A randomized controlled trial was performed on 170 first-time ST-elevation myocardial infarction (STEMI) patients undergoing pPCI. Before the procedure, patients in intervention group (n=85) received 300 mg dose of allopurinol and control group (n=85) received placebo. Then, for the next 28 days, 100 mg of allopurinol was given to allopurinol group and placebo to the other group. Patients were compared regarding the baseline characteristics, clinical findings and oneyear MACE.
Findings:
Our findings showed that patients receiving allopurinol had significantly longer doortoballoon time than the control group (60.76±19.38 vs. 50.06±16.38, P value: 0.001). During one year of followup, heart failure (HF), cerebrovascular event (CVA) and mortality occurred more frequently in allopurinol group but differences were not statistically significant. No significant difference was also seen between the two groups regarding MACE during followup or hospitalization (P=0.179, 0.330 respectively). KaplanMeier curve could not show a significant difference between the two groups in terms of mortality and MACE (P=0.317 and 0.128 respectively).
Conclusion:
According to findings of this trial allopurinol had no cardioprotective effect against adverse cardiovascular events or death in patients undergoing pPCI.
Trial Registration:
Identifier: IRCT20140512017666N2; https://irct.behdasht.gov.ir/.
Keywords: Allopurinol, Myocardial infarction, Primary percutaneous coronary intervention, Major adverse cardiovascular events
Copyright and License Information
© 2024 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
This study was funded by the Tabriz University of Medical Sciences, Tabriz, Iran. (Grant number:61539).
Introduction
Coronary artery disease (CAD) is the number one cause of mortality in most countries. CAD can lead to acute myocardial infarction (MI), especially ST elevation MI (STEMI), the most severe type.1 The World Health Organization (WHO) estimates that annually 7.3 million deaths worldwide occur due to MI.2 MI leads to significant long-term morbidity among survivors due to complications such as heart failure (HF) and raised risks of death, cerebrovascular event (CVA), and recurrent MI.3,4
It is essential to seek proper therapies to enhance the clinical outcomes of MI patients. The current standard treatment for acute STEMI is myocardial reperfusion, primarily done through primary percutaneous coronary intervention (pPCI.3
One of the enzymes involved in producing reactive oxygen species (ROS) is the xanthine oxidase (XO). ROS may lower the contractility of cardiac muscles, leading to HF.5 They are also implicated in ischemia-reperfusion injury, atherosclerosis, hypertension (HTN), and thrombosis.6 Allopurinol is a common XO inhibitor prescribed mainly to treat hyperuricemia. Allopurinol can prevent ROS production, unlike other antioxidants that reduce free radicals after production.7 The cardiovascular benefits of allopurinol have been suggested to occur through three fundamental mechanisms. The first action is increasing local tissue availability of oxygen and adenosine triphosphate by interfering with purine catabolism.8,9 The second one is reducing uric acid serum concentration, which is a pro-inflammatory factor.10,11 The third mechanism is inhibiting ROS generation, which promotes endothelial dysfunction and atherosclerosis plaque instability.12,13
Past considers investigating the impact of allopurinol on ventricular action, infarct size, reperfusion injury, and arrhythmia event have driven to conflicting results, and the results have not been collectively positive.5,14-16
Given the doubts about allopurinol’s benefits for preventing major adverse cardiovascular events (MACE) in cardiac patients, we aimed to evaluate the impact of allopurinol in patients with acute STEMI undergoing pPCI by comparing the incidence of HF, death, and CVA in patients enrolled in this randomized controlled trial (RCT).
Methods
This study was carried out at the Shahid Madani Heart Hospital in Tabriz, Iran from March 2020 to March 2021. The Tabriz University of Medical Sciences Ethics Committee approved the study protocol (IR.TBZMED.REC.1398.487), and written informed consent was obtained from all patients. Protocol of this trial was also registered in the Iranian Registry of Clinical Trials (IRCT: IRCT20140512017666N2, Registration date: 2019-10-15) and has been carried out in accordance with Declaration of Helsinki.
Study population and design
This RCT enrolled patients with STEMI who were admitted within 12 hours of symptom onset and underwent pPCI. Inclusion criteria required patients to be over 18 years old, have a confirmed diagnosis of first-time STEMI, and achieve successful pPCI, defined as post-angiographic stenosis of less than 25%. STEMI was defined as at least two continuous leads with ST-segment elevation of more than 2.5 mm in males under 40, 2 mm in men over 40, or 1.5 mm in women in leads V2-V3 and/or 1 mm in the remaining leads.14 Patients with any evidence of intimal dissection within 24 hours from admission were excluded. Other exclusion criteria were the presence or history of ventricular tachycardia or fibrillation, any kind of bundle block, previous HF or MI and history of revascularization, patients with severe valve disease, those who had contraindications for allopurinol use, patients with cardiogenic shock at the time of admission or history of previous CVA, and finally those who were candidates just for percutaneous old balloon angioplasty (POBA) were also excluded. We used the results of our previous study15 to determine the sample size. Considering α = 0.05 and a power of 80%, 80 patients in each group and a total of 160 patients were calculated for this study. One hundred eighty-four individuals were initially included in the study due to the potential of missing data and attrition. Random numbers were generated using the RANDLIST 1.2 software, and patients were divided into intervention and placebo groups based on sample size. Before the PCI, patients in each group received either a 300 mg dose of allopurinol or a placebo. Then, for the next 28 days, 100 mg of allopurinol was given to intervention group and placebo (which contained starch and was in the form of tablets) to control group. Aspirin 325 mg and clopidogrel 300 mg were given orally, along with weight-adjusted intravenous heparin, as part of the normal pretreatment protocol for pPCI. All pPCIs were performed out by expert interventional cardiologists using the standard technique, and none of the staff members in the catheterization lab were aware of the groups.
Laboratory and clinical measurements
Baseline findings were recorded in all patients including demographic information like age, gender, and medical history including HTN, diabetes mellitus (DM), hyperlipidemia, smoking, and family history of MI. On admission, venous blood samples were taken and placed in citrated tubes with ethylenediaminetetraacetate (EDTA) potassium salt additive. The hospital laboratory received and examined blood samples. To achieve laboratory results, a daily-calibrated automated Coulter CBC H1 counter was used. Measurements were performed for hemoglobin, creatinine, uric acid, and cardiac troponin I (cTnI). During the first 48 hours of hospitalization, the blood levels of cTnI were monitored every 6 hours, and their peak values were recorded. At admission and 90 minutes after pPCI, a standard 12-lead ECG was taken, and 20 milliseconds after the end of the QRS complex, the ST-E sum was measured for the affected leads. At the time of admission and 40 days following pPCI, the left ventricle ejection fraction (LVEF) was measured echocardiographically using the modified Simpson’s rule.
Follow-up
Throughout the one-year follow-up period, patients were monitored for occurrence of MACE. There were no patients lost to follow-up during the study period. Calls were made one, six, and 12 months after discharge to check on the patients. Re-hospitalizations due to HF and stroke at our hospital or other hospitals were detected and recorded through regular clinic visits.Diagnosis and assessment of HF in the hospital and clinic were done by an experienced cardiologist. Finally, patients’ information during one year of follow-up was collected and compared between the placebo and intervention groups.
Outcomes
MACE was defined as a composite of mortality, HF, and CVA during hospitalization and follow-up. The principal aim of the study was to evaluate the effect of allopurinol use on one-year MACE. Comparison of HF, CVA, and mortality separately between two groups during the follow-up period was the secondary outcome of this study.
Statistical analysis
All statistical analysis were conducted using SPSS (Version 21.0; IBM SPSS Corporation, Chicago, IL). The Kolmogorov-Smirnov test was used to verify the normal distribution assumption for continuous data. While qualitative data were provided as frequency and percent (%), quantitative data were shown as mean (SD). For continuous variables, an independent samples t-test was utilized. The chi-square test, as appropriate, was used to analyze categorical variables. Calculations were made to determine relative risks (RRs) and their confidence intervals (CIs). The Kaplan-Meier curve was used in survival analysis to compare the mortality and follow-up MACE between the two groups. P values < 0.05 were considered statistically significant.
Results
Of 184 patients diagnosed with anterior STEMI who were eligible to participate in this study, 14 patients were not enrolled according to the exclusion criteria as shown in Figure 1. Eventually, 170 patients were randomly divided into group A, the allopurinol-treated group (n = 85), or group B, the placebo group (n = 85). The baseline characteristics and demographics of the patients are shown in Table 1. The mean age of the whole population was 56.51 ± 11.66 years and the majority of the patients were men, however, there was no significant difference between the two groups regarding age and gender. Furthermore, the baseline features of patients including LVEF, hemoglobin, creatinine, uric acid, and cTnI concentrations did not statistically differ between the two groups. The most common risk factor in eligible participants was HTN (39.4%) followed by smoking (38.2%) and diabetes (20%). No significant difference was there in total ischemic time and ST elevation (P = 0.863, 0.172 respectively). The intervention group had significantly longer door-to-balloon time than the control group (60.76 ± 19.38 vs. 50.06 ± 16.38, P = 0.001). More than half of the patients (58.8%) had one vessel disease and almost all of them (98.8%) had TIMI (thrombolysis in MI) flow 3, but there was no significant difference between the two groups (P = 0.837 and 0.884, respectively). LVEF of the patients was compared after 40 days of pPCI and there was no significant difference between the two groups (mean: 37.79 ± 8.08 vs. 32.21 ± 4.89; P = 0.118). The mean left ventricular diastolic diameter (LVDD) was 51.05 ± 4.90 mm 40 days after MI. Approximately 70% of the patients had ST resolution of more than 50% after PCI although the difference between the two groups was not significant (P = 0.869).
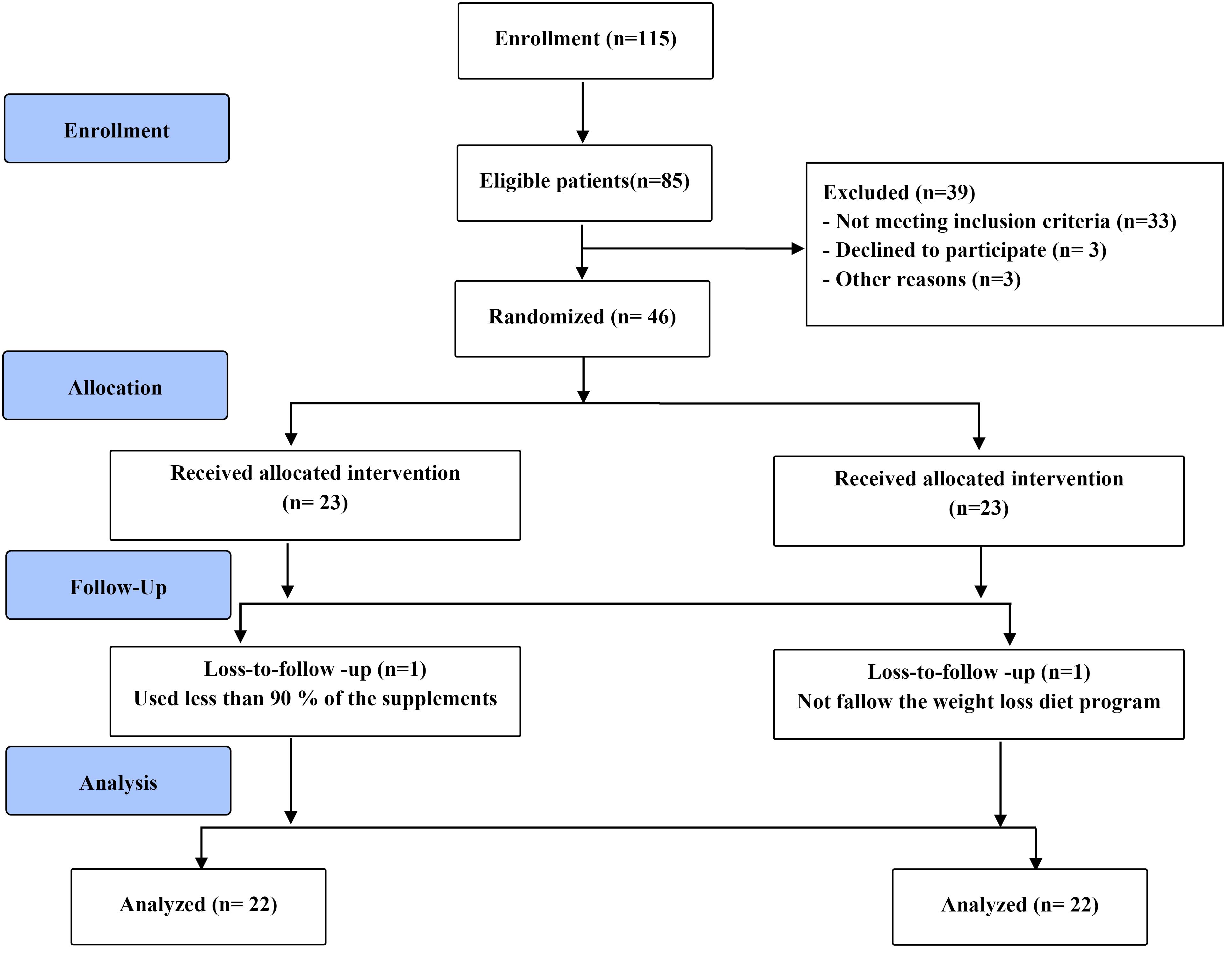
Figure 1.
Flow diagram of the study
.
Flow diagram of the study
Table 1.
Baseline characteristics of the patients
|
Finding
|
All patients (N=170)
|
Allopurinol (n=85)
|
Placebo (n=85)
|
P
value
|
| Age, year |
56.51 ± 11.66 |
55.23 ± 11.05 |
57.78 ± 12.16 |
0.154 |
| Gender |
|
|
|
0.193 |
| Male |
145 (85.3%) |
76 (89.4%) |
69 (81.2%) |
|
| Female |
25 (14.7%) |
9 (10.6%) |
16 (18.8%) |
|
| Ejection fraction (%) |
32.21 ± 4.89 |
31.76 ± 4.67 |
32.65 ± 5.09 |
0.241 |
| Hemoglobin (g/dL) |
15.01 ± 1.92 |
15.06 ± 1.98 |
14.97 ± 1.87 |
0.757 |
| Creatinine (mg/dL) |
1.02 ± 0.20 |
1.03 ± 0.19 |
1.02 ± 0.22 |
0.826 |
| Uric acid (mg/dL) |
5.15 ± 1.26 |
5.02 ± 1.29 |
5.28 ± 1.22 |
0.168 |
| Peak CTNI (ng/dL) |
14.56 ± 9.68 |
14.98 ± 10.50 |
14.13 ± 8.82 |
0.568 |
| Diabetes |
34 (20%) |
18 (21.2%) |
16 (18.8%) |
0.848 |
| Hypertension |
67 (39.4%) |
32 (37.6%) |
35 (41.2%) |
0.754 |
| Hyperlipidemia |
25 (14.7%) |
15 (17.6%) |
10 (11.8%) |
0.387 |
| Smoking |
65 (38.2%) |
37 (43.5%) |
28 (32.9%) |
0.207 |
| Familial history |
9 (5.3%) |
5 (5.9%) |
4 (4.7%) |
0.732 |
| TIT (min) |
273.59 ± 145.81 |
271.65 ± 153.74 |
275.53 ± 138.33 |
0.863 |
| DBT (min) |
55.44 ± 18.69 |
60.76 ± 19.38 |
50.06 ± 16.38 |
0.001 |
| Total STE (mm) |
13.74 ± 8.41 |
14.62 ± 8.81 |
12.85 ± 7.95 |
0.172 |
TIT: Total ischemic time, DBT: Door to balloon time, STE: ST elevation.
For continuous variables, an independent samples t-test was utilized. The chi-square test, as appropriate, was used to analyze categorical variables.
Post-angiographic outcomes of patients are shown in Table 2. As shown in the table, none of the measured outcomes differs significantly between the two groups.
Table 2.
Post-angiographic outcomes of patients
|
Finding
|
Total (N=170)
|
Allopurinol (n=85)
|
Placebo (n=85)
|
P
value
|
| EF 40 days after MI |
37.79 ± 8.08 |
36.82 ± 8.12 |
38.76 ± 7.97 |
0.118 |
| LVDD 40 days after MI |
51.05 ± 4.90 |
51.78 ± 3.52 |
50.32 ± 4.31 |
0.052 |
| STR > 50% |
116 (68.2%) |
59 (69.4%) |
57 (67.1%) |
0.869 |
| 1 vessel disease |
100 (58.8%) |
51 (60%) |
49 (57.6%) |
0.837 |
| 2 vessel disease |
44 (25.9%) |
21 (24.7%) |
23 (27.1%) |
|
| 3 vessel disease |
26 (15.3%) |
13 (15.3%) |
13 (15.3%) |
|
| TIMI flow |
|
|
|
0.884 |
| 1 |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
|
| 2 |
2 (1.1%) |
0 (0.0%) |
2 (2.3%) |
|
| 3 |
168 (98.8%) |
85 (100%) |
83 (97.6%) |
|
| MR |
|
|
|
0.454 |
| negative |
78 (45.9%) |
37 (43.5%) |
41 (48.2%) |
|
| + 1 |
60 (35.3%) |
32 (37.6%) |
28 (32.9%) |
|
| + 2 |
29 (17.1%) |
13 (15.3%) |
16 (18.8%) |
|
| + 3 |
2 (1.2%) |
2(2.4%) |
0 (0%) |
|
| + 4 |
1 (0.6%) |
1 (1.2%) |
0 (0%) |
|
| Stent diameter |
3.15 ± 0.43 |
3.15 ± 0.43 |
3.14 ± 0.44 |
0.958 |
| Stent length |
21.21 ± 6.91 |
21.36 ± 7.30 |
21.07 ± 6.54 |
0.782 |
EF: Ejection fraction, LVDD: Left ventricle diastolic diameter, STR: ST resolution, TIMI: Thrombolysis in myocardial infarction, MR: mitral regurgitation.
For continuous variables, an independent samples t-test was utilized. The chi-square test, as appropriate, was used to analyze categorical variables.
Table 3 shows the clinical outcomes of the whole population. Based on our findings 12 (14.1%) patients in the allopurinol group and 7 (8.2%) patients in the placebo group experienced in-hospital MACE (RR: 1.83, 95% CI: 0.68-4.90, P = 0.330). During the one-year follow-up, HF was the most common adverse event (16.5%) followed by CVA (3.5%) in all patients, with no significant difference between the two groups. Four (2.4%) patients died during the follow-up time and there was no statistically significant difference between the two groups regarding the follow-up mortality (P = 0.621). Thirty-four patients experienced MACE during the follow-up, of which 21 (24.7%) of them were in the allopurinol group (RR: 1.81, 95% CI: 0.84-3.92, P = 0.179).
Table 3.
In-hospital MACE and one-year outcomes of patients
|
Finding
|
All patients (N=170)
|
Allopurinol (n=85)
|
Placebo (n=85)
|
RR
|
95% CI
|
P
value
|
| In-hospital MACE |
19 (11.2%) |
12 (14.1%) |
7 (8.2%) |
1.83 |
0.68-4.90 |
0.330 |
| HF |
28 (16.5%) |
17 (20.0%) |
11 (12.9%) |
1.68 |
0.73-3.84 |
0.301 |
| CVA |
6 (3.5%) |
5 (5.9%) |
1 (1.2%) |
5.25 |
0.60-45.92 |
0.210 |
| Mortality |
4 (2.4%) |
3 (3.5%) |
1 (1.2%) |
3.07 |
0.31-30.15 |
0.621 |
| Follow-up MACE |
34 (20.0%) |
21 (24.7%) |
13 (15.3%) |
1.81 |
0.84-3.92 |
0.179 |
MACE: major adverse cardiovascular events, HF: heart failure, CVA: cerebrovascular accident; RR, relative risk.
Multivariate analysis was done to determine the RR and P values.
Also, we performed a survival analysis to compare the mortality and MACE during the one-year follow-up between the two groups. As shown in Figure 2 and Figure 3 there was no significant difference between the two groups in terms of both mortality and MACE (P = 0.317 and 0.128, respectively).
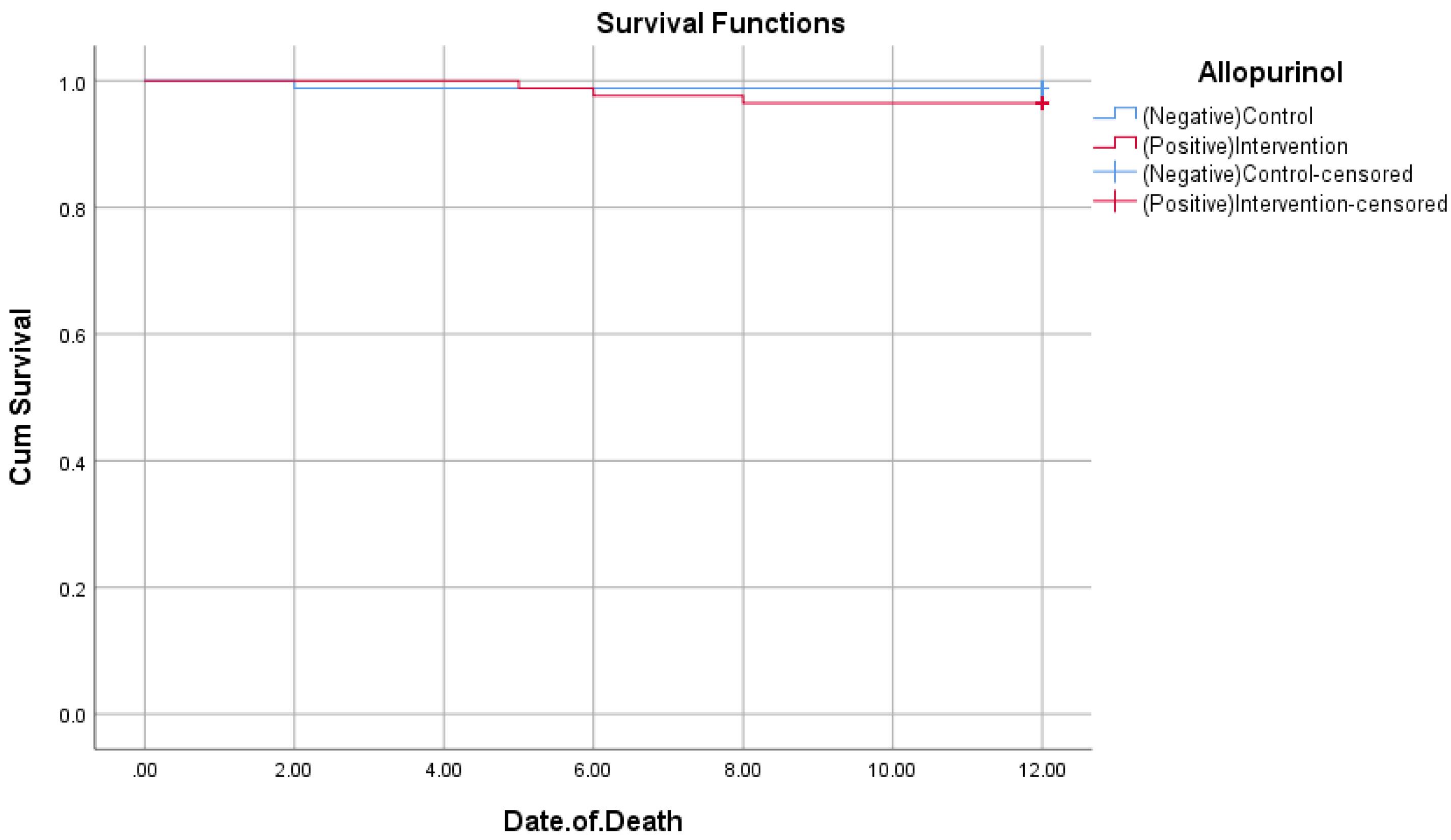
Figure 2.
Kaplan-Meier curve for patients’ mortality (P = 0.317)
.
Kaplan-Meier curve for patients’ mortality (P = 0.317)
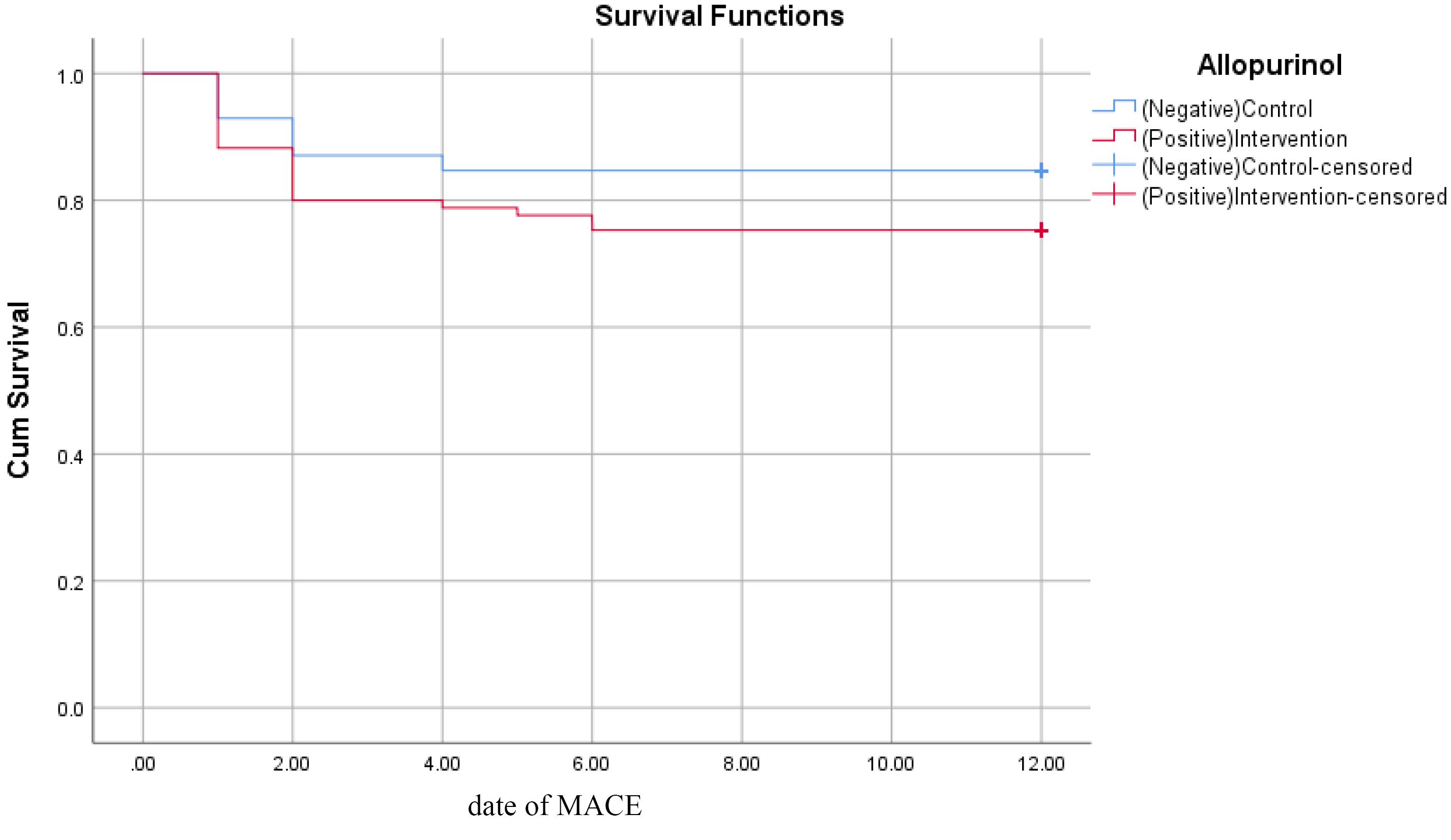
Figure 3.
Kaplan-Meier curve for follow-up MACE (P = 0.128)
.
Kaplan-Meier curve for follow-up MACE (P = 0.128)
Discussion
In the present study, we evaluated the effect of allopurinol on the incidence of MACE, HF, CVA, and mortality in adult patients undergoing pPCI. We did not find any significant difference in in-hospital MACE and in HF, CVA, mortality separately or follow-up MACE between the group receiving allopurinol after pPCI and the placebo group. While rates of these complications were higher in the intervention group, none of the differences were statistically significant. There were no significant differences in baseline characteristics between two groups except door to balloon time (DBT), which was longer in the intervention group. The results of this research are supported by the context of literature within this field. In a meta-analysis by Bredemeier et al16 65 RCTs for MACE and 74 RCTs for death were included in the analysis of the efficacy of purine-like XO inhibitors for prevention of cardiovascular events and mortality in patients from different settings. This study failed to establish a significant effect for these medications in the prevention of cardiovascular events and mortality. Data of off-treatment follow-up periods were excluded from this study. However, another meta-analysis by Singh et al.17 analyzing allopurinol efficacy in reducing the incidence of MI following coronary artery bypass grafting (CABG) showed cardioprotective effects for allopurinol. However, only six studies published before 2000 satisfied the eligibility criteria of this meta-analysis. Ullah et al18 addressed a similar question by analyzing nine studies, of which five of them were the same as the study of Singh et al.17 Ullah et al18 could not find any long-term benefits in terms of secondary prevention of acute coronary syndrome or mortality in patients undergoing CABG. Considering the findings of previous studies reporting the potential cardioprotective effects of allopurinol against MACE or death, no consensus has been established and many newer studies have reported contradictory findings on survival-increasing effects for allopurinol in cardiovascular disorders.19-21 However, allopurinol has been reported to positively affect HTN,22-24 endothelial function,25-27 left ventricular mass,28-30 rate of progression of kidney disease,31 and perinatal hypoxia-induced free radical formation.32,33 In a study by Goicoechea et al,31 107 patients with chronic kidney disease were divided into two groups and followed up for five years. They found that long-term treatment with allopurinol can slow the rate of progression of kidney disease and reduce cardiovascular risk in patients with chronic kidney disease. Kanbay et al34 analyzed 11 trials that treated HF patients with uric acid-lowering agents, which were allopurinol in most of the studies. They found these agents were associated with worse mortality outcomes and higher LVEF but did not significantly affect hospitalizations. In another study by Weisman et al35 mortality and cardiovascular outcomes in an allopurinol-treated diabetes cohort of the elderly were assessed. Allopurinol was found to reduce all-cause mortality and cardiovascular events in this group of patients. Singh et al36 also found similar results in patients with gout and diabetes. It can be concluded that certain patient populations or patients with specific comorbidities might benefit from allopurinol and studies so far have not been able to uniformly prove the positive effects of allopurinol on the survival of the general population with cardiovascular disorders. Therefore, further research on the survival-increasing effects of allopurinol needs to focus on specified populations to determine which populations and subsets of patients benefit the most from adding allopurinol to their medication regimen. Besides the uncertainty of around prescribing allopurinol to patients, there is also no consensus on whether patients will benefit from higher doses. Some studies have found higher dosages to be harmful and some beneficial. Bredemeier et al16 found that the use of high doses of allopurinol ( > 300 mg/d), especially with furosemide, may lead to worse cardiovascular outcomes than treatment with lower dosages. Rekhraj et al28 found high-dose allopurinol to regress left ventricle hypertrophy, and improve endothelial function in patients with ischemic heart disease. Rajendra et al37 also found that high-dose allopurinol (600 mg/d) can decrease vascular tissue oxidative stress in optimally treated CAD patients. Therefore, the dosage of allopurinol should also be taken into account when interpreting its effect and indication.
Strengths of this study include its similar patient groups and accurate follow-up, as well as blinding of the patients and assessors. On the other hand, this study occurred in a single center and only included patients from the Caucasian race, which can make generalization of our findings to other races difficult. In addition, we only followed up the patients for a year and outcomes might have changed if there was a longer follow-up period. Therefore, future studies need to recruit more patients and follow them for longer, however, it is suggested to do so in patient populations with specific characteristics or comorbidities that might benefit from the effects of xanthine inhibitors.
Conclusion
Our findings show that allopurinol, despite possessing beneficial features in the treatment of other diseases, might not help patients undergoing pPCI with overall survival or occurrence of adverse outcomes. Therefore, it is recommended to thoroughly review the possible benefits of allopurinol before considering it as a possible choice for improving survival.
Competing Interests
Authors declare that there is no competing of interests.
Ethical Approval
All methods were carried out following relevant guidelines and regulations. The data is anonymized, and no information regarding patients’ identities was disclosed. The study was performed in accordance with the Helsinki Declaration. The protocol of this study was reviewed by the Ethics Committee of Tabriz University of Medical Sciences (ID: IR.TBZMED.REC.1398.487) and registered in the Iranian Registry of Clinical Trials (identifier: IRCT20140512017666N2).
Acknowledgements
We thank the staff of the Shahid Madani hospital for their cooperation, who helped us gather the data used for this analysis.
References
- Malakar AK, Choudhury D, Halder B, Paul P, Uddin A, Chakraborty S. A review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol 2019; 234(10):16812-23. doi: 10.1002/jcp.28350 [Crossref] [ Google Scholar]
- Chadwick Jayaraj J, Davatyan K, Subramanian SS, Priya J. Epidemiology of myocardial infarction. In: Pamukçu B, ed. Myocardial Infarction. IntechOpen; 2019. 10.5772/intechopen.74768.
- Fröhlich GM, Meier P, White SK, Yellon DM, Hausenloy DJ. Myocardial reperfusion injury: looking beyond primary PCI. Eur Heart J 2013; 34(23):1714-22. doi: 10.1093/eurheartj/eht090 [Crossref] [ Google Scholar]
- Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest 2013; 123(1):92-100. doi: 10.1172/jci62874 [Crossref] [ Google Scholar]
- Ju C, Lai RW, Li KH, Hung JK, Lai JC, Ho J. Comparative cardiovascular risk in users versus non-users of xanthine oxidase inhibitors and febuxostat versus allopurinol users. Rheumatology (Oxford) 2020; 59(9):2340-9. doi: 10.1093/rheumatology/kez576 [Crossref] [ Google Scholar]
- Brieger K, Schiavone S, Miller FJ Jr, Krause KH. Reactive oxygen species: from health to disease. Swiss Med Wkly 2012; 142:w13659. doi: 10.4414/smw.2012.13659 [Crossref] [ Google Scholar]
- Sica DA, Carter B, Cushman W, Hamm L. Thiazide and loop diuretics. J Clin Hypertens (Greenwich) 2011; 13(9):639-43. doi: 10.1111/j.1751-7176.2011.00512.x [Crossref] [ Google Scholar]
- Okafor ON, Farrington K, Gorog DA. Allopurinol as a therapeutic option in cardiovascular disease. Pharmacol Ther 2017; 172:139-50. doi: 10.1016/j.pharmthera.2016.12.004 [Crossref] [ Google Scholar]
- Puddu P, Puddu GM, Cravero E, Vizioli L, Muscari A. Relationships among hyperuricemia, endothelial dysfunction and cardiovascular disease: molecular mechanisms and clinical implications. J Cardiol 2012; 59(3):235-42. doi: 10.1016/j.jjcc.2012.01.013 [Crossref] [ Google Scholar]
- Zhao L, Cao L, Zhao TY, Yang X, Zhu XX, Zou HJ. Cardiovascular events in hyperuricemia population and a cardiovascular benefit-risk assessment of urate-lowering therapies: a systematic review and meta-analysis. Chin Med J (Engl) 2020; 133(8):982-93. doi: 10.1097/cm9.0000000000000682 [Crossref] [ Google Scholar]
- van der Pol KH, Wever KE, Verbakel M, Visseren FLJ, Cornel JH, Rongen GA. Allopurinol to reduce cardiovascular morbidity and mortality: a systematic review and meta-analysis. PLoS One 2021; 16(12):e0260844. doi: 10.1371/journal.pone.0260844 [Crossref] [ Google Scholar]
- Weisman A. Associations Between Allopurinol and Cardiovascular and Renal Outcomes in Diabetes. University of Toronto; 2020.
- Guedes M, Esperança A, Pereira AC, Rego C. What is the effect on cardiovascular events of reducing hyperuricemia with allopurinol? An evidence-based review. Rev Port Cardiol 2014; 33(11):727-32. doi: 10.1016/j.repc.2014.06.002 [Crossref] [ Google Scholar]
- Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018; 39(2):119-77. doi: 10.1093/eurheartj/ehx393 [Crossref] [ Google Scholar]
- Separham A, Ghaffari S, Najafi H, Ghaffari R, Ziaee M, Babaei H. The impact of allopurinol on patients with acute ST elevation myocardial infarction undergoing thrombolytic therapy. J Cardiovasc Pharmacol 2016; 68(4):265-8. doi: 10.1097/fjc.0000000000000409 [Crossref] [ Google Scholar]
- Bredemeier M, Lopes LM, Eisenreich MA, Hickmann S, Bongiorno GK, d’Avila R. Xanthine oxidase inhibitors for prevention of cardiovascular events: a systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc Disord 2018; 18(1):24. doi: 10.1186/s12872-018-0757-9 [Crossref] [ Google Scholar]
- Singh TP, Skalina T, Nour D, Murali A, Morrison S, Moxon JV. A meta-analysis of the efficacy of allopurinol in reducing the incidence of myocardial infarction following coronary artery bypass grafting. BMC Cardiovasc Disord 2018; 18(1):143. doi: 10.1186/s12872-018-0881-6 [Crossref] [ Google Scholar]
- Ullah W, Khanal S, Khan R, Basyal B, Munir S, Minalyan A. Efficacy of allopurinol in cardiovascular diseases: a systematic review and meta-analysis. Cardiol Res 2020; 11(4):226-32. doi: 10.14740/cr1066 [Crossref] [ Google Scholar]
- Suissa S, Suissa K, Hudson M. Effectiveness of allopurinol in reducing mortality: time-related biases in observational studies. Arthritis Rheumatol 2021; 73(9):1749-57. doi: 10.1002/art.41710 [Crossref] [ Google Scholar]
- Harzand A, Tamariz L, Hare JM. Uric acid, heart failure survival, and the impact of xanthine oxidase inhibition. Congest Heart Fail 2012; 18(3):179-82. doi: 10.1111/j.1751-7133.2011.00262.x [Crossref] [ Google Scholar]
- Kelkar A, Kuo A, Frishman WH. Allopurinol as a cardiovascular drug. Cardiol Rev 2011; 19(6):265-71. doi: 10.1097/CRD.0b013e318229a908 [Crossref] [ Google Scholar]
- Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. Jama 2008; 300(8):924-32. doi: 10.1001/jama.300.8.924 [Crossref] [ Google Scholar]
- Soletsky B, Feig DI. Uric acid reduction rectifies prehypertension in obese adolescents. Hypertension 2012; 60(5):1148-56. doi: 10.1161/hypertensionaha.112.196980 [Crossref] [ Google Scholar]
- Kostka-Jeziorny K, Uruski P, Tykarski A. Effect of allopurinol on blood pressure and aortic compliance in hypertensive patients. Blood Press 2011; 20(2):104-10. doi: 10.3109/08037051.2010.532323 [Crossref] [ Google Scholar]
- Yelken B, Caliskan Y, Gorgulu N, Altun I, Yilmaz A, Yazici H. Reduction of uric acid levels with allopurinol treatment improves endothelial function in patients with chronic kidney disease. Clin Nephrol 2012; 77(4):275-82. doi: 10.5414/cn107352 [Crossref] [ Google Scholar]
- Kanbay M, Huddam B, Azak A, Solak Y, Kadioglu GK, Kirbas I. A randomized study of allopurinol on endothelial function and estimated glomular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin J Am Soc Nephrol 2011; 6(8):1887-94. doi: 10.2215/cjn.11451210 [Crossref] [ Google Scholar]
- Alem MM. Allopurinol and endothelial function: a systematic review with meta-analysis of randomized controlled trials. Cardiovasc Ther 2018; 36(4):e12432. doi: 10.1111/1755-5922.12432 [Crossref] [ Google Scholar]
- Rekhraj S, Gandy SJ, Szwejkowski BR, Nadir MA, Noman A, Houston JG. High-dose allopurinol reduces left ventricular mass in patients with ischemic heart disease. J Am Coll Cardiol 2013; 61(9):926-32. doi: 10.1016/j.jacc.2012.09.066 [Crossref] [ Google Scholar]
- Szwejkowski BR, Gandy SJ, Rekhraj S, Houston JG, Lang CC, Morris AD. Allopurinol reduces left ventricular mass in patients with type 2 diabetes and left ventricular hypertrophy. J Am Coll Cardiol 2013; 62(24):2284-93. doi: 10.1016/j.jacc.2013.07.074 [Crossref] [ Google Scholar]
- Kao MP, Ang DS, Gandy SJ, Nadir MA, Houston JG, Lang CC. Allopurinol benefits left ventricular mass and endothelial dysfunction in chronic kidney disease. J Am Soc Nephrol 2011; 22(7):1382-9. doi: 10.1681/asn.2010111185 [Crossref] [ Google Scholar]
- Goicoechea M, Garcia de Vinuesa S, Verdalles U, Verde E, Macias N, Santos A. Allopurinol and progression of CKD and cardiovascular events: long-term follow-up of a randomized clinical trial. Am J Kidney Dis 2015; 65(4):543-9. doi: 10.1053/j.ajkd.2014.11.016 [Crossref] [ Google Scholar]
- Kaandorp JJ, Benders MJ, Schuit E, Rademaker CM, Oudijk MA, Porath MM. Maternal allopurinol administration during suspected fetal hypoxia: a novel neuroprotective intervention? A multicentre randomised placebo-controlled trial. Arch Dis Child Fetal Neonatal Ed 2015; 100(3):F216-23. doi: 10.1136/archdischild-2014-306769 [Crossref] [ Google Scholar]
- Kaandorp JJ, van den Broek MP, Benders MJ, Oudijk MA, Porath MM, Bambang Oetomo S. Rapid target allopurinol concentrations in the hypoxic fetus after maternal administration during labour. Arch Dis Child Fetal Neonatal Ed 2014; 99(2):F144-8. doi: 10.1136/archdischild-2013-304876 [Crossref] [ Google Scholar]
- Kanbay M, Afsar B, Siriopol D, Dincer N, Erden N, Yilmaz O. Effect of uric acid-lowering agents on cardiovascular outcome in patients with heart failure: a systematic review and meta-analysis of clinical studies. Angiology 2020; 71(4):315-23. doi: 10.1177/0003319719897509 [Crossref] [ Google Scholar]
- Weisman A, Tomlinson GA, Lipscombe LL, Perkins BA, Hawker GA. Association between allopurinol and cardiovascular outcomes and all-cause mortality in diabetes: a retrospective, population-based cohort study. Diabetes Obes Metab 2019; 21(6):1322-9. doi: 10.1111/dom.13656 [Crossref] [ Google Scholar]
- Singh JA, Ramachandaran R, Yu S, Curtis JR. Allopurinol use and the risk of acute cardiovascular events in patients with gout and diabetes. BMC Cardiovasc Disord 2017; 17(1):76. doi: 10.1186/s12872-017-0513-6 [Crossref] [ Google Scholar]
- Rajendra NS, Ireland S, George J, Belch JJ, Lang CC, Struthers AD. Mechanistic insights into the therapeutic use of high-dose allopurinol in angina pectoris. J Am Coll Cardiol 2011; 58(8):820-8. doi: 10.1016/j.jacc.2010.12.052 [Crossref] [ Google Scholar]