Biomed adv. 2(3):104-130.
doi: 10.34172/bma.29
Review Article
miRNA-Loaded stem cell-derived exosomes in neuroregeneration: Current insights and future perspectives
Ayse Melis Ozdemir Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, # 
Helin Lara Senoglu Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, # 
Mohammadreza Dastouri Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – review & editing, * 
Author information:
Department of Medical Biology, Faculty of Medicine and Genetics, Ankara Medipol University, Ankara, Turkey
#Both authors contributed equally to this work and share first authorship.
Abstract
Summary
The use of exosomes derived from stem cells as microRNA (miRNA) delivery vehicles for neuroregenerative medicine is an area that is gaining increasing attention. Their biocompatible natural structure and ability to effectively interact with target cells make these nanometric vesicles a candidate of interest for modulating nerve repair mechanisms. The scalability of exosome production and the standardization of isolation methods still require further development. Furthermore, miRNAs are being increasingly investigated due to their roles in regulating gene expression and neuronal survival and regeneration through various pathways in which they participate. This review examines current information on the biogenesis and molecular profile of stem cell-derived exosomes, as well as various endogenous and bioengineering-based strategies for miRNA loading. Besides, mechanisms including ESCRT-dependent and ESCRT-independent pathways, as well as other physical and chemical processes, are explained in terms of specificity and effectiveness. Furthermore, this review assesses the vast therapeutic promise of miRNA-loaded exosomes in enhancing neurogenesis, regulating inflammation, preserving synaptic plasticity, and inhibiting apoptosis. Special attention is also paid to experimental findings in various neurodegenerative disorders such as Alzheimer’s, Parkinson’s, amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), Huntington’s, and spinal cord injuries, and translational challenges in these areas. Finally, emerging approaches, such as personalized exosome-based therapies, gene editing tools like CRISPR, and integrated therapeutic platforms, are discussed in this review as elements that may shape future developments.
Keywords: Exosomes, microRNA (miRNA), Neuroregeneration, Stem cell therapy, Neurodegenerative diseases
Copyright and License Information
© 2025 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
None.
Introduction
Cells communicate with each other by exchanging signals through various pathways. Cells that send signals about their functioning and other cells through autocrine, paracrine, or endocrine stimulation primarily transmit these signals in extracellular vesicles (EVs), especially exosomes.1 In stem cell treatments, the goal is to improve stem cells that can proliferate and differentiate into various cells by utilizing their paracrine factors instead of using them directly. However, research shows that exosomes are the main responsible for the paracrine effect. In other words, applying treatment with exosomes secreted by stem cells instead of using them directly makes the treatment easier, more applicable, and safer.2
Stem cell exosomes can be obtained from a wide variety of stem cell types, such as embryonic, induced pluripotent, hematopoietic, mesenchymal, endothelial, and neural stem cells.3 Stem cell exosomes are stable, so they can remain intact in the body, exhibit cell-specific effects, and escape the immune system.4 These make stem cell exosomes more advantageous than using stem cells directly. One of the important components carried by these exosomes is microRNAs (miRNA). Differentiation of neural progenitor cells, neurogenesis, synaptic reorganization, and neuron-glia cell interactions are neural regeneration pathways that are spontaneously found in our body and regulated by miRNAs. At the same time, studies are also being conducted to provide neural regeneration by taking advantage of the important role of miRNAs in cell programming with bioengineering.5 All of these point to the pioneering role of miRNAs in both physiological and therapeutic neural regeneration.6
Dysfunctions of miRNAs are closely related to cancer, genetic diseases, infections, inflammations, cardiovascular diseases, neurodegenerative diseases, and many other pathologies. This relationship has increased research on treatment approaches through miRNA in recent years. In studies, miRNA-like molecules or miRNA antagonists are preferred according to the molecular process of the relevant disease.7,8
The expression of miRNAs changes after neurological traumas such as brain injuries, spinal cord injuries, and peripheral nerve injuries. Changes in miRNA expression regulate neural regeneration through post-transcriptional modifications.9 For this reason, a thorough understanding of these miRNAs and an investigation of their therapeutic potential may provide an effective treatment opportunity for nerve injuries in the future.
It is challenging for miRNAs to pass directly through the cell membrane because both the cell membrane and miRNAs are negatively charged; In addition, miRNAs have high renal clearance, may have problems in passing from the endosome to the cytoplasm, may be destroyed by nucleases, may be phagocytosed, may trigger the immune system (especially the reticuloendothelial system), and may cause other side effects.10,11 For this reason, miRNAs must be taken into the cell through various transfer mechanisms to benefit from miRNAs. These mechanisms are divided into two viral and nonviral transfer mechanisms. Although viral mechanisms are advantageous in terms of high efficiency and stable miRNA expression, they carry serious risks, including immune response, toxicity, and mutation. Transfer by viral mechanisms is carried out through retroviruses, lentiviruses, adenoviruses, and adeno-associated viruses. Each vector has its areas of use, advantages, and disadvantages.8,12
Nonviral transfer mechanisms can be physical, such as gene guns, electroporation, or hydrodynamic injection, or chemical, including lipid-based or polymer-based systems and inorganic carriers. In nonviral transfer mechanisms, unlike viral mechanisms, much less toxicity and immunogenicity are encountered; the surfaces of the carriers can be customized and have flexible carrying capacity. However, their carrying efficiency is also lower compared to viral transfer mechanisms.8,11 Despite all these disadvantages, their safety makes nonviral mechanisms more preferred in the clinic.13 For this reason, researchers have concentrated their efforts on optimizing nonviral transfer mechanisms. In these studies, exosome is an excellent carrier thanks to their advantages14 (Figure 1).
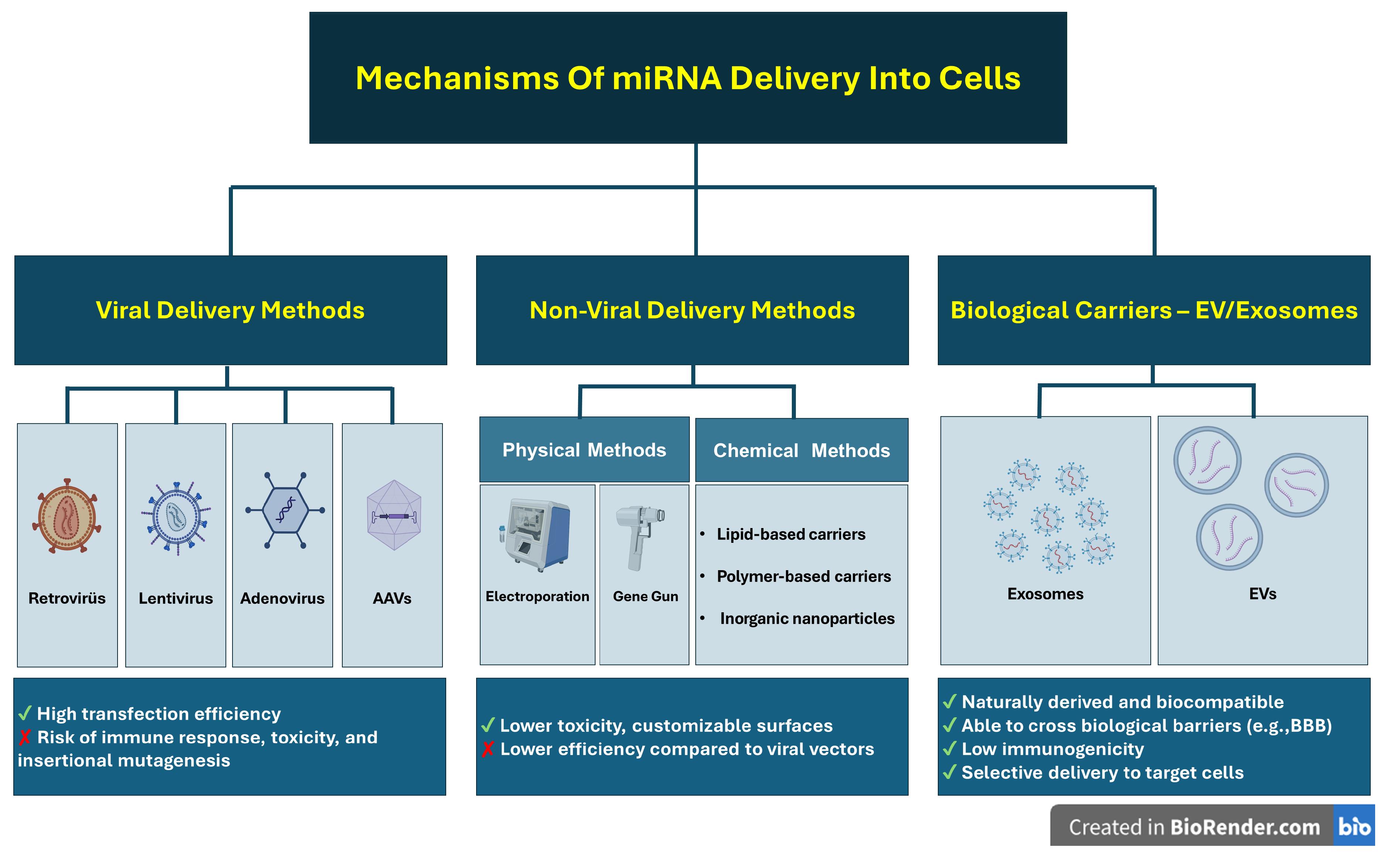
Figure 1.
Mechanisms of miRNA delivery into cells
.
Mechanisms of miRNA delivery into cells
Exosomes are considered excellent carriers for miRNAs due to their unique biocompatibility, ability to cross biological barriers (especially the blood-brain barrier [BBB]),15 interact with the extracellular matrix and trigger a response in cells in the microenvironment,16 high stability, natural origin, selectivity,17 and long-distance intercellular communication.18 Despite the difficulties encountered in their isolation, loading, and targeting processes, exosomes have demonstrated their drug delivery potential in different animal models.19 Moreover, it has been confirmed that exosomes carry miRNAs and enhance their activities in various ways, such as triggering apoptosis, supporting angiogenesis, or regulating lipid metabolism.20 The fact that this situation is not encountered in traditional treatments has made exosome-based miRNA delivery more advantageous. For all these reasons, miRNAs loaded into stem cell exosomes are being investigated as a strong treatment option in both neurodegenerative diseases and other diseases for which no curative treatment has yet been found. In this study, we have compiled the current studies on miRNAs loaded into stem cell exosomes.
Biogenesis and composition of stem cell-derived exosomes
Exosome formation and release mechanisms
Exosomes are small EVs derived from late endosomes and released as a result of the fusion of multivesicular bodies with the plasma membrane.21 Unlike apoptotic bodies and microvesicles, exosomes are formed by the release of intraluminal vesicles (ILVs) into the external environment by ESCRT-dependent or -independent mechanisms.22,23 Throughout this process, Rab5-to-Rab7 conversion, and subsequently the role of Rab11, Rab27a/b, and Rab35 proteins, lead to the release of exosomes beyond the cell.24,25 Exosomes were originally assumed to be cellular trash, but were afterwards known to be actively engaged in intercellular exchange.21
Molecular cargo: proteins, lipids, and nucleic acids
Exosomes harbor biomolecules such as proteins, lipids and nucleic acids, and participate in physiological and pathological processes and are also candidate biomarkers or drugs.26,27 The composition of exosomes are particularly dependent on the condition of the source cell.28,29 Loading of cargo onto exosomes for therapeutic use can be carried out at biogenesis or ex vivo.30 Databases like ExoCarta that contain data on content of exosomes offer isolation and characterization methods as well as content analysis methods.31,32
Mechanisms of miRNA loading into stem cell exosomes
Endogenous (natural) loading mechanisms
Endogenous (natural) loading mechanisms refer to the cell’s intrinsic ability to selectively package miRNAs into EVs and exosomes during their biogenesis. These processes are tightly regulated and involve multiple pathways, such as ESCRT-dependent and ESCRT-independent sorting, RNA-binding protein (RBP)-mediated selective loading, sequence and structure-specific miRNA motifs, ceramide and lipid raft-associated pathways, and the involvement of Argonaute proteins and the miRNA-induced silencing complex (miRISC) complex. Additionally, cellular conditions like stress, microenvironmental cues, and differentiation states significantly influence these natural loading mechanisms, shaping the functional cargo profile of EVs and exosomes.
miRNA loading through ESCRT-dependent and independent pathways
Endosomal sorting complexes required for transport (ESCRT) is a membrane remodeling tool found in all life forms and transcends evolutionary boundaries.33 ESCRTs were first discovered and named for their essential role in recognizing and directing ubiquitin-labeled multi-pillar vesicle body (MVB) cargoes into MVB vesicles.34 ESCRT complexes have a wide range of functional functions,33 including classical functions such as MVB biogenesis, budding of HIV and other viruses, and membrane separation during cytokinesis, as well as more recently discovered functions such as microvesicle and exosome biogenesis, micro- and macroautophagy, removal of damaged nuclear pore complexes, and nuclear envelope remodeling.35
The ESCRT protein machinery plays a role in the transformation of endosomes into multivesicular bodies. ESCRT consists of five different protein complexes. These are ESCRT-0, -I, -II, -III and AAA ATPase Vps4. ESCRT-0 recognizes proteins marked with ubiquitin, which is a “send for destruction” signal, and accumulates them in the endosomal membrane. Thus, the MVB pathway is initiated. ESCRT-I binds to both ESCRT-0 and ubiquitinated cargo, more effectively sequesters the protein it receives from ESCRT-0, and also interacts with ESCRT-II to advance the process. ESCRT-II then calls the ESCRT-III complex and initiates polymerization. ESCRT-III forms membrane invaginations into the cytoplasm in a topologically reversed direction from classical surface budding, trapping cargo and playing a role in cell membrane formation, constriction, closure and rupture. Finally, the AAA ATPase Vps4 disassembles ESCRT-III, allowing the ESCRT system to be reused.36-40
A comprehensive RNA interference (RNAi) screen in the HeLa cell line identified seven ESCRT proteins that affect exosome secretion: ESCRT-0 proteins Hrs and TSG101, ESCRT-I protein STAM1 increased exosome release; ESCRT-III and its related proteins CHMP4C, VPS4B, VTA1 and ALIX reduced exosome release. The functions of Hrs, TSG101, STAM1 and VPS4B of these proteins were confirmed by further studies.41 It has been shown that the absence of ALIX also disrupts exosome biogenesis both in vivo and in vitro.42
The general knowledge is that the ESCRT protein complex is required for the formation of ILVs. However, as a result of some studies, it has been understood that ESCRT-independent pathways can also provide ILV and thus exosome formation. ILV formation in mammals occurs through both ESCRT-dependent and ESCRT-independent pathways.43 Tetraspins, SIMPLE/LITAF protein, and ceramides are examples of ESCRT-independent mechanisms.21,25,44
To give an example of the functioning of ESCRT-independent pathways, tetraspins, which are transmembrane proteins such as CD9, CD63, and CD81, organize cargoes on the membrane into clusters and facilitate the budding of ILVs into the membrane. Ceramide and cholesterol-rich membrane regions also recruit certain proteins such as flotillin and LC3 and support ILV formation. Flotillin attracts the GTPase Rab31 to the MVB. Rab31, which is phosphorylated and activated by EGFR (epidermal growth factor receptor), initiates ILV budding by binding to the SPFH domain of flotillin proteins. Thus, receptors such as EGFR accumulate in MVBs containing CD63, an exosome marker.45,46
Selective miRNA transport mediated by RBPs
Certain RBPs, such as hnRNPA2B1 and YBX1, recognize specific miRNA sequence motifs and mediate their selective incorporation into exosomes. This mechanism confers sequence specificity to the miRNA loading process, allowing for regulated and targeted cargo sorting. In 2013, Villarroya-Beltri and colleagues demonstrated that the RBP hnRNPA2B1 plays a critical role in the selective loading of miRNAs into exosomes by recognizing specific sequence motifs known as EXOmotifs. Their study showed that the sumoylation of hnRNPA2B1 is essential for its interaction with these motifs, thereby modulating miRNA sorting. Mutational analysis of these motifs and silencing of hnRNPA2B1 significantly altered the exosomal miRNA profile, highlighting a regulated and sequence-specific mechanism of miRNA packaging.47
miRNA sequence motifs and structural recognition signals
Certain miRNAs contain short sequence motifs such as the EXO motif that are specifically recognized by carrier proteins. These structural elements play a critical role in guiding miRNAs into exosomes and contribute to the selectivity of exosomal cargo loading.
In a 2022 study conducted by Garcia-Martin and colleagues, the authors demonstrated that specific sequence motifs within miRNAs namely EXOmotifs and CELLmotifs play a critical role in determining whether these molecules are exported into small sEVs or retained within the parent cell. Their experiments further revealed that the RBPs Alyref and Fus mediate the selective export of miRNAs that harbor potent EXOmotifs, such as CGGGAG. This research provides valuable insights into the molecular mechanisms of miRNA trafficking and identifies potential strategies for refining RNA-based therapeutic delivery.48
Ceramide and lipid raft associated loading mechanisms
Ceramide facilitates the inward budding of endosomal membranes by inducing membrane curvature, thereby promoting the sorting of miRNAs into ILVs. Additionally, lipid rafts contribute to the cargo loading process by modulating protein–lipid interactions, further influencing miRNA packaging within EVs.
In a 2008 study, Trajkovic and colleagues investigated the mechanisms underlying exosome formation within multivesicular endosomes. They discovered that ceramide, a sphingolipid, facilitates the inward budding of endosomal membranes, leading to the generation of ILVs that are later secreted as exosomes. Notably, this process was found to be independent of the ESCRT (endosomal sorting complex required for transport) machinery. Their experiments showed that purified exosomes were enriched in ceramide, and inhibiting neutral sphingomyelinases significantly reduced exosome release. These findings highlight a ceramide-dependent, ESCRT-independent pathway for exosome biogenesis, offering new insights into the molecular mechanisms of vesicular trafficking and intercellular communication.49
The role of Argonaute proteins and the miRISC complex in miRNA loading
Components of the miRISC complex, such as Ago2, play a key role in guiding miRNAs bound to their target proteins within the cell toward exosomal packaging. This process contributes significantly to the regulation of miRNA activity. In 2009, Gibbings and colleagues demonstrated that MVBs are not merely vesicular transport stations, but also play an active role in the intracellular regulation of miRNA activity. Through their experimental observations, they showed that key components of the miRISC, particularly Argonaute 2, accumulate in association with MVBs. This finding suggested that the trafficking of miRISC through the endosomal system may be essential for modulating gene silencing efficiency, revealing a deeper layer of regulatory control within the miRNA machinery.50
miRNA loading influenced by cellular stress, microenvironment, and differentiation status
Microenvironmental changes such as hypoxia and oxidative stress have been shown to alter both the composition of secreted exosomes and their miRNA cargo. This dynamic modulation is thought to reflect an adaptive mechanism, contributing to cellular defense and regenerative responses. In their 2022 study, Jiang et al demonstrated that hypoxic conditions significantly alter both the quantity and molecular composition of exosomes secreted by cells. Their findings highlight the role of HIF-1α in regulating exosomal cargo particularly miRNAs and proteins thereby reshaping the biological activity of these vesicles. Exosomes released under hypoxia were shown to influence angiogenesis, cellular proliferation, and immune responses in recipient cells. These observations underscore the clinical relevance of hypoxia-induced exosomal changes, especially in the context of tumor microenvironments and regenerative medicine.51
Preconditioning and genetic modification of stem cells
Stem cells can be genetically modified to induce the overexpression of specific target miRNAs. Through this approach, these miRNAs are naturally incorporated into exosomes during the endogenous biogenesis process, offering a strategic method to engineer exosomal content for therapeutic applications. In 2016, Wang et al. demonstrated that mesenchymal stem cells (MSCs) can be genetically modified to overexpress the miRNA let-7c, which is subsequently packaged into exosomes via the cell’s endogenous biogenesis pathways. These engineered exosomes were then administered in a model of renal fibrosis, where they effectively delivered let-7c to damaged renal tissue. The treatment significantly reduced fibrotic gene expression and improved kidney histology. This study highlights the therapeutic potential of MSC-derived exosomes as targeted miRNA delivery vehicles in fibrotic diseases.52
Exogenous (artificial/bioengineering-based) loading mechanisms
Exogenous (artificial or bioengineering-based) loading mechanisms represent a set of deliberate strategies designed to introduce specific miRNAs into eEVs or exosomes. These methods bypass natural sorting processes and include various techniques such as chemical transfection (e.g., using lipofectamine), passive incubation, freeze-thaw cycles, electroporation, and sonication (Figure 2) Each approach offers distinct advantages and limitations in terms of efficiency, vesicle integrity, and cargo specificity, and their selection often depends on the experimental goal and sensitivity of the biological system.
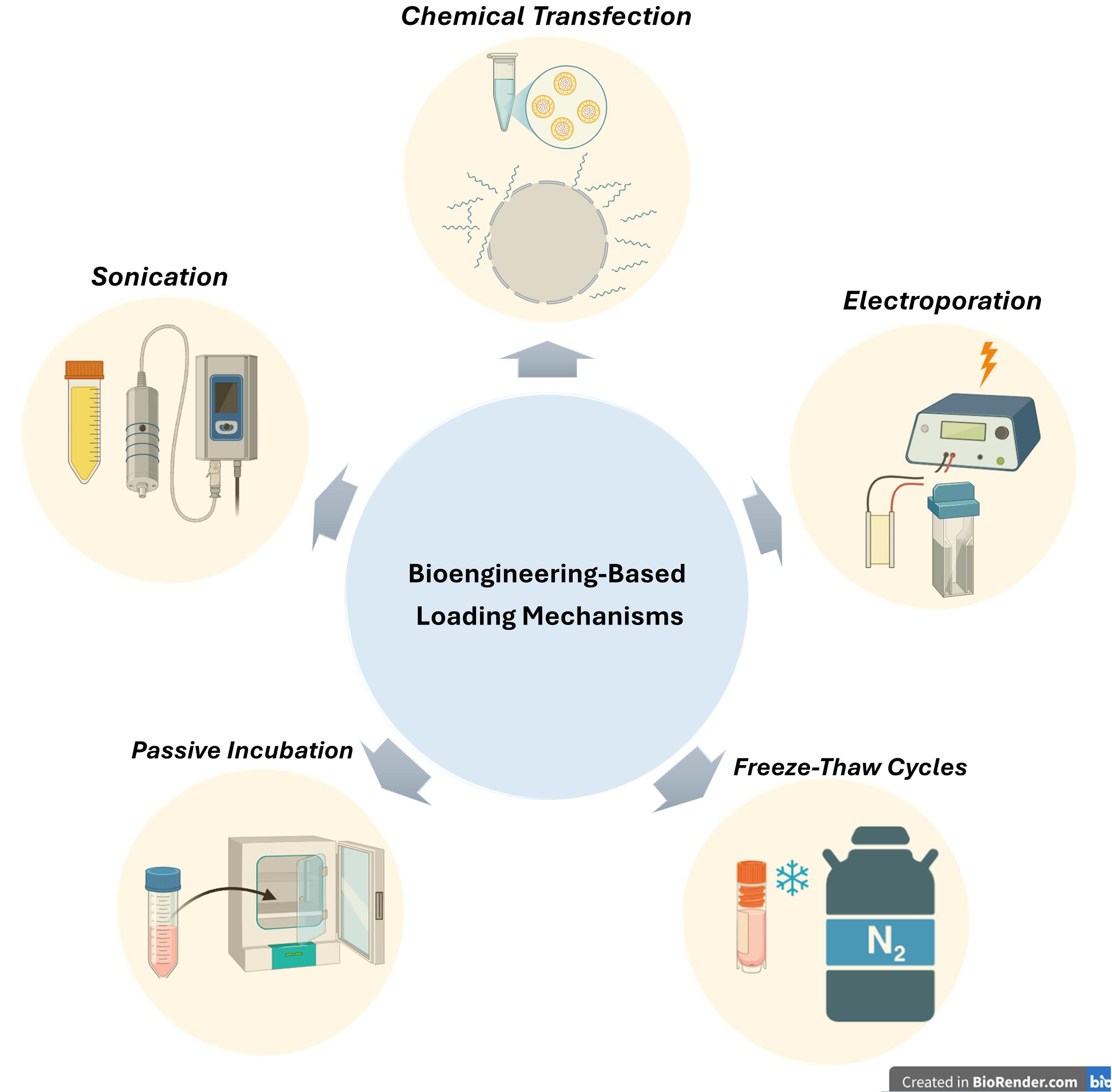
Figure 2.
Exogenous (artificial/bioengineering-based) loading mechanisms
.
Exogenous (artificial/bioengineering-based) loading mechanisms
Loading via electroporation
Electroporation involves applying brief electrical pulses to create transient pores in the exosome membrane, facilitating the entry of miRNAs into the vesicles. While this method achieves a high loading efficiency, it may compromise the structural integrity of the exosomal membrane. In 2019, Pomatto et al investigated the use of electroporation to load antitumor miRNAs into plasma-derived EVs. They optimized electrical pulse parameters to maximize loading efficiency while preserving vesicle integrity and natural cargo. Their results demonstrated that electroporation significantly enhanced miRNA encapsulation compared to passive incubation, protecting the miRNAs from degradation and maintaining effective delivery to target cells.53
Loading via chemical transfection
Chemical transfection has emerged as a commonly used method for loading miRNAs into exosomes and other EVs. In this approach, lipid- or polymer-based reagents such as Lipofectamine form complexes with synthetic miRNAs, facilitating their incorporation into isolated vesicles. This technique is relatively straightforward and widely accessible in laboratory settings, making it attractive for experimental use. However, it often results in lower loading efficiency compared to endogenous methods and carries the risk of co-isolating residual transfection reagents or free RNA complexes. Despite these limitations, chemical transfection remains a useful tool for functionalizing EVs, particularly in early-stage research focused on therapeutic RNA delivery.
In their 2013, Shtam et al demonstrated that Lipofectamine could be used to load exogenous small interfering RNA (siRNA) into exosomes, establishing a chemically driven method for exosomal cargo engineering. By transfecting siRNA into isolated exosomes, they were able to deliver functional siRNA to human cells in vitro, resulting in measurable gene silencing. This work positioned Lipofectamine-mediated transfection as an early and accessible approach for manipulating exosomal content, though the authors noted the importance of controlling for potential contamination from free siRNA-lipid complexes.54
Loading via passive incubation
In the passive incubation method, exosomes are incubated with synthetic miRNAs under controlled temperature and time conditions, allowing spontaneous association or diffusion of miRNAs into the vesicles. Although this technique generally results in low loading efficiency, it is favored for its simplicity, safety, and non-invasive nature. Due to the minimal disruption of exosomal structure, passive incubation is considered a gentle strategy, particularly suitable for preliminary studies where preserving the integrity of vesicles is crucial. In 2021, Brossa and colleagues conducted a study using passive coincubation as a method to load synthetic miR-145 into EVs derived from human liver stem cells. By gently incubating the miRNA with isolated EVs, they successfully achieved miRNA incorporation without damaging the vesicles’ structure. Although this technique has lower efficiency compared to active methods, it offers a simple and non-disruptive way to engineer EVs. Their results showed enhanced anti-tumor activity of the miRNA-loaded EVs, highlighting passive incubation as a valuable and practical strategy for therapeutic miRNA delivery.55
Loading via freeze-thaw cycles
In this approach, exosomes are mixed with synthetic miRNAs and subjected to repeated freeze-thaw cycles. The process temporarily increases membrane permeability, allowing miRNAs to pass into the vesicles during the thawing phase. While this technique may compromise membrane integrity, it has shown some effectiveness under specific conditions and remains a simple and accessible loading strategy.In 2020, Lee and colleagues conducted a study where they utilized freeze-thaw cycles to load miR-140 into exosomes, aiming to enhance their therapeutic efficacy. This method allowed the successful incorporation of miRNA without complex procedures, and the exosome-miR-140 complexes significantly promoted chondrogenic differentiation of bone marrow-derived stem cells. Their findings highlight freeze-thaw loading as a simple yet promising strategy for cartilage repair and regenerative medicine applications.56
Loading via sonication
In this method, ultrasonic vibrations are used to temporarily disrupt the exosomal membrane, allowing for the entry of miRNAs into the vesicles. While this technique can enhance loading efficiency, the application of high-energy waves may compromise membrane integrity, potentially affecting the structural and functional stability of the exosomes. Nonetheless, under optimized conditions, sonication remains a viable strategy for therapeutic cargo delivery and is frequently explored in experimental studies. In 2016, Lamichhane and colleagues conducted a pioneering study where they used sonication as an active loading strategy to incorporate small RNAs (siRNA and miRNA) into EVs. By applying short ultrasonic pulses, they temporarily disrupted the EV membranes, allowing RNA molecules to enter without significantly compromising vesicle integrity. The engineered EVs successfully delivered the RNA cargo to recipient cells, leading to oncogene knockdown, including effective suppression of HER2 expression. This work highlighted sonication as a promising and controllable technique for functionalizing EVs in therapeutic applications, especially in gene silencing strategie.57
Neuroregenerative effects of miRNA-loaded exosomes
Promotion of neurogenesis and neuronal differentiation
Neurogenesis, the formation of new neurons, is rare in the adult mammalian brain. Three regions are exceptions: the olfactory bulb, the dentate gyrus of the hippocampus, and the hypothalamus.58,59
In humans, these regions are not identical. For example, neurogenesis in the olfactory bulb does not occur in humans. Also, unlike other mammals, neurogenesis occurs in humans’ striatum, the center of movement, reward, and decision-making.60 The regulation of neurogenesis is regulated by many different factors, from environmental stimuli to hormones, neurotransmitters to nutrition, and medication use to age. Disruption of this rather delicate balance is associated with neurodegenerative disorders such as dementia and temporal lobe epilepsy, as well as psychiatric disorders such as schizophrenia and major depression. Therefore, the existence of neurogenesis in the adult brain carries the light of being an effective treatment for the mentioned diseases.58,61
Pluripotent human embryonic stem cells (hESCs) obtained from early blastocyst stage embryos can produce an unlimited number of neurons62 and transform into neural progenitor cells. Neural progenitor cells provide neuronal differentiation through cellular signaling pathways from the external environment.63 This way, even dopaminergic neurons can be obtained.64 The fact that hESCs can remain intact and pluripotent in culture for a long time, exhibit functional properties, and behave similarly to primary cells makes them a potential cell source for studies on neuronal differentiation.65
Considering the consequences of even a minor disruption in these two vital mechanisms in humans, the importance of regulating intercellular communication and ensuring a healthy neurogenic niche and healthy neurogenesis is understood. This communication is provided not only through classical pathways but also through exosomes and the miRNAs they carry. These exosomal miRNAs play a role in regulating the activity of genes related to neurogenesis and neuronal differentiation in target cells.66,67 For example, MSCs specifically load miRNA into exosomes during the body’s normal functioning and thus regulate angiogenesis, which plays a critical role in neurogenesis and its efficiency68 (Table 1).
Table 1.
Primary studies on miRNA-mediated effects on neurogenesis and neuronal differentiation
|
miRNA loaded into exosomes
|
The cell of origin of the exosome
|
Result
|
Disease
|
Reference
|
| miR-193a |
F11(Neural progenitor cells) |
Neuronal differentiation and neurogenesis |
Not specifically mentioned. |
Oh69 |
| miR-21a |
Neural stem/progenitor cells (NPCs) |
Neuronal differentiation and neurogenesis by activating Akt and Wnt signaling pathways |
Not specifically mentioned. |
Ma et al70 |
| miR-9 |
Neural stem cells (NSCs) |
Neuronal differentiation, neurogenesis, and neuronal and glial cell maturation by targeting the Hes1 gene |
Diseases that cause the loss of nerve cells |
Yuan et al71 |
| miR-124, miR-132, miR-133b, miR-218, miR-9, miR-34b, miR-34c, miR-135a2 |
Epiblast-derived stem cells (EpiSCs) |
Dopaminergic neuron differentiation through pathways regulating stem cell pluripotency, such as the FoxO signaling pathway, DA synapses, Wnt signaling pathway, GABAergic synapses, and neurotrophin signaling pathways |
Neurodegenerative diseases such as Parkinson's disease |
Jin et al72 |
| miR-7a-5p |
Hippocampal NSCs |
Inhibition of neuronal differentiation mediated by the Tcf12 transcription factor |
Diseases affecting hippocampal neurogenesis |
Sun et al73 |
| miR-125b |
PC12 |
Neuronal differentiation |
Not specifically mentioned. |
Takeda and Xu74 |
| miR-21 ve miR-19b |
EVs from differentiated PC12 cells and hMSCs |
Enhancement of neuronal differentiation and reduction of neuronal apoptosis by suppressing PTEN expression |
Spinal cord injury |
Xu et al75 |
| miR-126 |
MSC |
Neurogenesis, angiogenesis, and functional recovery through the inhibition of SPRED1 and PIK3R2 genes |
Spinal cord injury |
Huanget al76 |
| miR-206-3p antagomir |
MSC |
Hippocampal neurogenesis via activation of the BDNF/TrkB signaling pathway. |
Alzheimer’s disease |
Peng et al77 |
Axonal growth and synaptic plasticity enhancement
The mechanisms that enable recovery after central nervous system injuries are not yet fully understood, but it is known that axon regrowth plays a significant role in the recovery process. This is because loss of function after neurological damage is usually due to the severing of axon connections rather than the death of neurons.78 Axon re-growth is essential in neuronal plasticity and regeneration, forming new neural circuits and functional recovery. Functional recovery requires myelination, synapse formation, and pruning of incorrect connections in addition to axon re-growth.79 In the adult brain, plasticity can direct new synapse formation and provide larger-scale circuit changes. This feature shows the uniqueness of our brain in terms of self-regulation and development.80 In addition, the permanent nature of plasticity shows that changes at the axonal level can last a lifetime,81 ensuring the continuity of improvements provided by plasticity.
Although creating a microenvironment suitable for healing with stem cells has a significant place in treatment studies for neurological injuries that are common and cause serious consequences, such as spinal cord injury (SCI), the fact that stem cells have limited direct effects and that they perform their effects through paracrine signals poses a problem in stem cell research. This is where exosomes and the miRNAs they carry have emerged as a relatively new and promising strategy.15
The miRNAs carried in exosomes can affect the neuron’s structure, function, and genetic program by suppressing or altering gene expression in the target cell. miRNAs whose regulation changes in disease states have various effects on neurons82 (Table 2).
Table 2.
Listing of major studies on the effects of miRNAs from various cells on axonal growth and synaptic plasticity
|
miRNA loaded into exosomes
|
The cell of origin of the exosome
|
Result
|
Disease
|
Reference
|
| miR-26a |
Astrocyte |
By suppressing the ctdsp2 gene, there is potential to affect the morphology and synaptic transmission of neurons. |
Neurological diseases |
Lafourcade et al82 |
| Ex-miR-133b⁺ |
Mesenchymal stromal cell |
By reducing the level of RABEPK protein, neurite remodeling, neural plasticity, and functional recovery occur. |
Stroke |
Xin et al83 |
| miR-20b-5p |
LPS-activated BV2 microglia |
By suppressing the PTEN gene and activating the PI3K-AKT pathway, neuronal process outgrowth and synaptic improvement occur in the presence of mild hypothermia. |
Traumatic brain injury |
Wang et al84 |
| miR-17-92 cluster |
Mesenchymal stromal cell |
With the suppression of the PTEN gene, activation of Akt, mTOR, and GSK-3β leads to axonal growth, neurogenesis, and oligodendrogenesis. |
Stroke |
Xin et al85; Zhang et al86 |
| miRNA-21 |
Repair Schwann cells (rSCs) |
By suppressing the PTEN gene and activating the PI3K-Akt pathway, axon growth occurs." |
Peripheral nerve injury |
López-Leal et al87 |
| miR-133b |
Adipose tissue-derived stem cell |
Axonal regeneration through RhoA/ROCK reduction and ERK increase, along with CREB and STAT3 regulation. |
Spinal cord injury |
Shen & Cai15 |
| miR-494 |
Mouse MSC |
Neurofilament renewal |
Spinal cord injury |
Huang et al88 |
| EXmiR-132-3p |
MSC |
Suppression of RASA1 protein increases Ras activity, enhances phosphorylation of Akt and GSK-3β proteins, and decreases p-Tau levels. Consequently, synaptic plasticity, neurite outgrowth, and branching occur. |
Vascular dementia |
Ma et al89 |
| miR-206-3p antagomir |
MSC |
Neurite outgrowth and synaptic plasticity through activation of the BDNF/TrkB signaling pathway. |
Alzheimer’s disease |
Peng et al77 |
| miR-126-3p |
Mouse brain endothelial cells called bEnd.3 |
Neurite growth |
Ischemia / Reperfusion Injury |
Gao et al90 |
| miR-17-92 + |
MSC |
By suppressing PTEN and activating the PI3K/Akt/mTOR pathway, compensatory neuroplasticity, axon elongation, and myelin remodeling are promoted. |
Stroke |
Xin et al91 |
| miR-5121 |
Microglia |
By suppressing the RGMa gene, neurite growth and synaptic improvement occur. |
Traumatic brain injury |
Zhao et al92 |
Anti-inflammatory and neuroprotective properties
In neurological disorders, secondary damage, such as neuroinflammation following the initial damage, can lead to serious neurodegeneration. Chronic neuroinflammation, a common feature of central nervous system diseases, increases neuronal deaths due to microglia activation and pro-inflammatory cytokine release, leading to disease progression.93
The disruption of the tasks undertaken by microglia, which are neuroimmune cells, in the field of brain homeostasis and neuroprotection in the CNS94 is effective in this situation because microglia activation can suppress inflammation in neurodegenerative disorders and sometimes increase it.95 Therefore, anti-inflammatory substances have become a new target in treating these disorders.
MSCs, the most commonly used cell type in neurological diseases, can affect inflammation. One of the most important functions of MSCs in providing this targeted anti-inflammatory effect is the exosomes they secrete and the contents they carry.96 Exosomes affect the course of diseases by regulating gene transcription of the miRNAs they carry.97 For example, against the changes in miRNA profiles in neurological disorders like stroke, traumatic brain injury, Alzheimer’s, and MS, miRNAs contained in exosomes have potential in the treatment of neurodegenerative disorders with their healing properties98,99 (Table 3).
Table 3.
Important studies on the anti-inflammatory and neuroprotective effects of miRNAs derived from various cells
|
miRNA loaded into exosomes
|
The cell of origin of the exosome
|
Result
|
Disease
|
Reference
|
| miR-124-3p |
Microglia |
By inhibiting the mTOR signaling pathway, it exerts anti-inflammatory, neuroprotective effects and supports neuronal repair and synaptic plasticity. |
Traumatic brain injury |
Mavroudis et al100 |
| miRNA-223-3p |
Microglia treated with sinomenine |
It suppresses the NLRP3-mediated pyroptosis mechanism. |
Chronic cerebral hypoperfusion |
Yang et al101 |
| miR-137 |
M2-type microglia |
It exerts a neuroprotective effect against ischemia-reperfusion injury by targeting the Notch1 gene. |
Ischemic brain injury |
Zhang et al94 |
| miR-672-5p |
M2-type microglia |
By inhibiting the AIM2/ASC/caspase-1 signaling pathway, it suppresses neuronal pyroptosis and supports functional recovery. |
Traumatic spinal cord injury |
Zhou et al102 |
| miR-338-5p |
Bone marrow stem cell (BMSCs) |
When overexpressed in exosomes, Rap1 increases due to Cnr1 inhibition, thereby activating the PI3K/Akt1 pathway and providing neuroprotective effects. |
Spinal cord injury |
Zhang et al103 |
| miR-21-5p |
Gingival MSCs stimulated with TNF-α |
Suppression of PDCD4 reduces cell death and protects retinal neurons. |
Retinal ischemia / reperfusion injury |
Yu et al104 |
| miR-124 |
M2-type microglia |
By inhibiting USP14, it exerts anti-inflammatory effects and protects neurons against ischemia. |
Ischemia / reperfusion injury |
Song et al105 |
| miR-182-5p |
Astrocytes treated with berberine |
By binding to Rac1, it suppresses neuroinflammation. |
Stroke |
Ding et al106 |
Modulation of apoptosis and cell survival pathways
Exosomes and the miRNAs they contain, which form the basis of their use in cellular therapies for disorders that cause both acute neurological disorders, such as traumatic brain injury, and serious problems, such as long-term disability, can have effects that support neurological recovery, such as intrinsic neurogenesis and neurite growth. They also play a role in the pathophysiological process.107 In a like manner, depending on whether the exosomes have different cargo, exosomes may provide different effects in neurodegenerative diseases, such as Alzheimer’s, Huntington’s, and Parkinson’s, which are becoming increasingly common as the world are changing as well as we are living longer. Exosomal miRNAs play a large role in the progression of these diseases, responding to oxidative stress that may ultimately lead to neuronal apoptosis through the processes they activate.108,109 As can be understood from studies on Parkinson’s110 the reason for the widespread effects of such neurodegenerative disorders is exosomes and the contents they carry. For this reason, exosomes equipped with engineering methods, such as exosomes derived from miRNA-loaded modified iPSC, are promising treatment methods.111 (Table 4)
Table 4.
Primary studies on the effects of miRNAs from various cells on neuronal apoptosis and modulation of cell survival pathway
|
miRNA loaded into exosomes
|
The cell of origin of the exosome
|
Result
|
Disease
|
Reference
|
| miR-223 |
MSC |
By inhibiting the PTEN gene, it activates the PI3K/Akt signaling pathway, thereby protecting neurons from apoptosis. |
Hypoxia on Alzheimer model cells |
Wei et al112 |
| miR-134 |
BMSC |
By suppressing caspase-8, encoded by the CASP8 gene, it reduces apoptosis in oligodendrocytes damaged by ischemia. |
Ischemic stroke |
Xiao et al113 |
| miR-374-5p |
Neural stem cell |
By targeting the SKT-4 gene, it initiates autophagic flux and suppresses apoptosis. |
Spinal cord injury |
Zhang & Han114 |
| miR-542-3p |
MSC |
By inhibiting TLR4, it reduces apoptosis and suppresses the inflammatory response. |
Cerebral infarction |
Cai et al115 |
| miR-672-5p |
M2-type microglia |
By inhibiting the AIM2/ASC/caspase-1 pathway, it halts neuronal pyroptosis. |
Spinal cord injury |
Zhou et al102 |
| miR-124-3p |
BV2 microglia |
By suppressing FIP200, it prevents excessive autophagy-induced neuronal death by halting autophagy. |
Traumatic brain injury |
Li et al116 |
| miR-129-5p |
MSC |
By reducing the elevation of HMGB1 and its associated TLR4, it induces an anti-inflammatory response, alleviates neuronal damage, and suppresses epilepsy-related abnormal neurogenesis. |
Status epilepticus |
Liu et al117 |
Therapeutic applications in neurodegenerative diseases
Neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis (ALS), and Huntington’s disease (HD) are characterized by progressive neuronal loss and currently lack effective curative treatments. In recent years, increasing attention has been directed toward miRNAs and exosomes as promising therapeutic agents due to their ability to modulate gene expression and mediate intercellular communication in the central nervous system. miRNAs can regulate key molecular pathways involved in neuroinflammation, apoptosis, synaptic plasticity, and neuronal survival, while exosomes offer a natural, biocompatible delivery system capable of crossing the BBB. This section explores the emerging therapeutic applications of miRNAs and exosome-based strategies in the context of neurodegenerative disorders, highlighting preclinical findings, delivery challenges, and potential for clinical translation. (Figure 3)
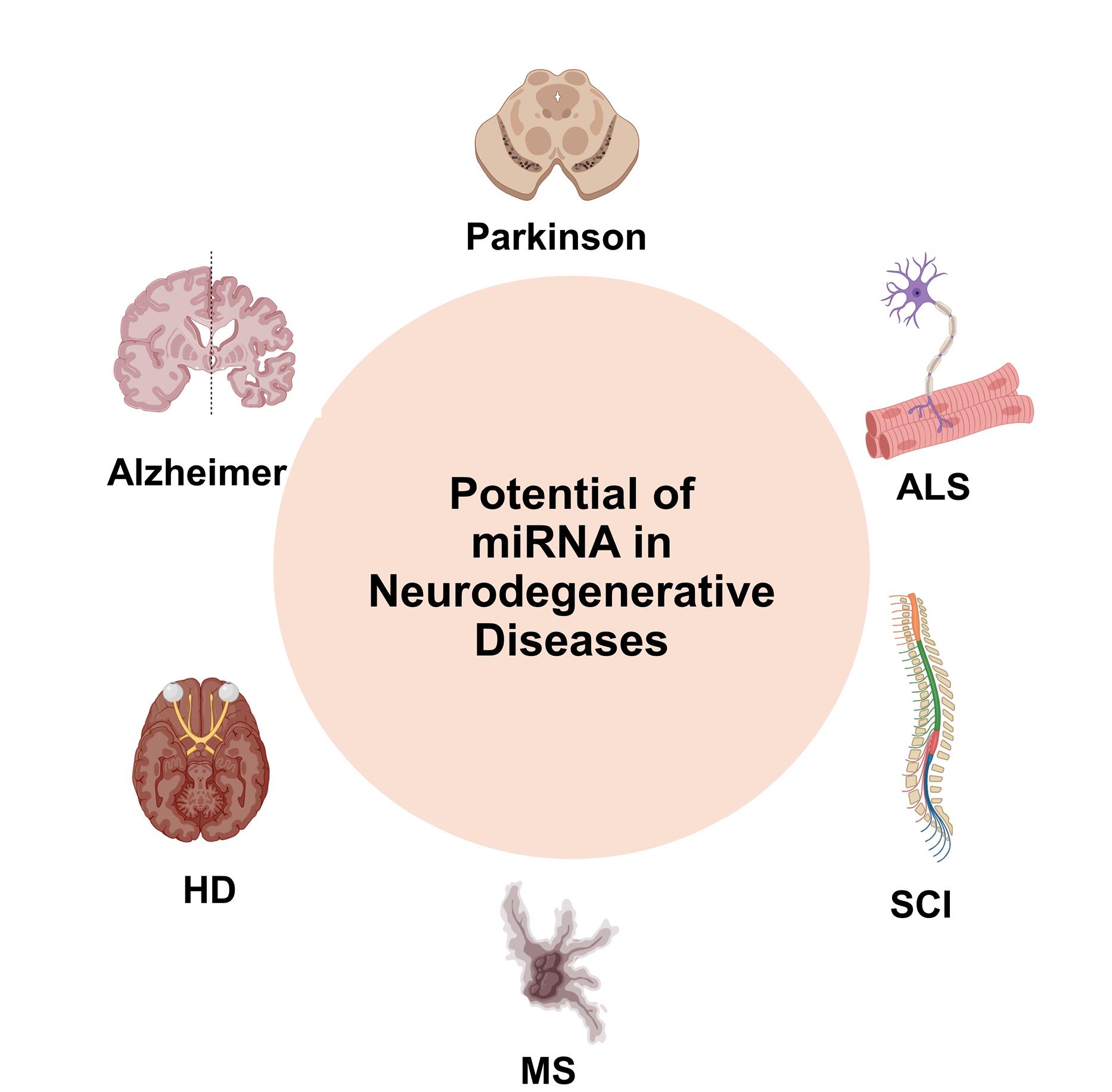
Figure 3.
Potantial of miRNA in neurodegenerative diseases
.
Potantial of miRNA in neurodegenerative diseases
Alzheimer’s disease: targeting amyloid-beta and tau pathologies
Alzheimer’s disease is usually seen in sixth decade of life, people aged 65 and over. Only 5% of people with Alzheimer’s disease are younger than 65. Alzheimer’s disease accounts for approximately ⅔ of dementia cases. Alzheimer’s disease is a neurodegenerative disease that begins insidiously and causes cognitive dysfunction. Cognitive dysfunctions include memory loss, difficulty in comprehension, difficulty in concentrating, difficulty in speaking, difficulty in reasoning and judging an event. Alzheimer’s disease does not directly cause death, but other complications resulting from this disease can lead to the patient’s condition ending in death.118
The lesions that occur in the brains of Alzheimer’s patients are senile plaques and intracellular neurofibrillary tangles (NFT). Amyloid-β is an essential and major protein found in senile plaques. Tau protein is the most significant protein component of NFT. In other words, when the brains of Alzheimer’s patients are examined, amyloid-β and tau protein accumulation are seen. At the same time, when the brains of Alzheimer’s patients are examined radiologically, they are seen with widened sulci and narrowed gyrus. When the brains of Alzheimer’s patients are analyzed in terms of size, it is seen that they are approximately 8-15% smaller than the brains of a healthy individual of the same age.119
Alzheimer’s disease is an insidious and progressive disease that focuses on episodic memory. In the early stages of the disease, amnestic mild cognitive impairment criteria can be seen. In the later stages, difficulties in multitasking, loss of self-confidence, and inability to use location-orientation perception correctly affect daily life. As the disease progresses, cognitive difficulties begin to affect daily life activities, and after this stage, this patient may be diagnosed with Alzheimer’s dementia. After the diagnosis of Alzheimer’s dementia is made, the patient is now dependent on the bed and someone to help them with their daily tasks. In the more advanced stages of the disease, behavioral changes, impaired mobility, hallucinations, and seizures may occur. Death occurs approximately 8.5 years after diagnosis.120
Alzheimer’s has recently become a major public health problem, but its treatment is poor. Recent studies are attempting to treat both cognitive impairment and behavioral symptoms. Treatment, primarily focused on cholinergic therapy, aims to enhance mental functions, and facilitate daily living activities. Future directions in the diagnosis and treatment of patients with Alzheimer’s are application of functional brain imaging techniques for early diagnosis and evaluation of treatment, development of new drug classes that work on different neurotransmitter systems for the treatment of symptoms such as cognitive deficit and behavioral disorders121 (Table 5).
Table 5.
Recent studies on the effects of Alzheimer’s-related miRNAs
|
miRNA
|
Disease
|
Expression change
|
Result
|
Reference
|
| miR-153 |
Alzheimer |
Decreased |
Amyloid-β precursor protein and endogenous Amyloid Precursor Protein (APP) expression are downregulated. |
Long et al122 |
| miR-339-5p |
Alzheimer |
Decreased |
miR-339-5p inhibits BACE1 expression and Aβ production in the human glioblastoma cell line U373. |
Long et al123 |
| miR-34a |
Alzheimer |
Increased |
miRNA-34a plays a role in regulating Sirt1, amyloid precursor protein, and Aβ levels. |
Gunnels124 |
| miR-34a |
Alzheimer |
Increased |
It has been reported that miR-34a levels are higher in patients compared to the control group. |
Chen et al125 |
| miR-135a |
Alzheimer |
Decreased |
miR-135a levels are lower in patients compared to the control group. |
Chen et al125 |
| miR-146a |
Alzheimer |
Increased |
miRNA-146a is involved in the activation of HSV-1, a key component of the arachidonic acid cascade. |
Hill et al126 |
| miR-146a |
Alzheimer |
Increased |
Endogenous Lrp2 protein expression levels are significantly inhibited by miRNA-146a in human SH-SY5Y cells. |
Zhang et al127 |
| miR-125b |
Alzheimer |
Decreased |
Serum levels of miR-125b are decreased in patients with Alzheimer’s disease. |
Galimberti et al128 |
| miR-26b |
Alzheimer |
Decreased |
Although the ROC curve suggests that miR-26b is unsuitable as a biomarker, it plays a role in the pathogenesis of the disease. |
Galimberti et al128 |
| miR-223 |
Alzheimer |
Decreased |
miR-223 is a major regulator of glutamate receptor expression. |
Galimberti et al128 |
| miR-485 |
Alzheimer |
Increased |
In the brains of Alzheimer’s patients, SV2A expression is decreased. miR-485 regulates the presynaptic protein SV2A. |
Cohen et al129 |
It has been suggested that Alzheimer’s disease is caused by excessive accumulation of amyloid-β peptide (Aβ). Aβ is generated from the Aβ precursor protein (APP) through a series of events in which many soluble fragments are released. Overexpression of APP leads to Alzheimer’s disease, with specific genetic abnormalities being rare presentations of the disease. Given the central role of APP and Aβ in Alzheimer’s pathology, it is necessary to elucidate the various mechanisms that regulate the physiological expression of APP to identify new drug targets to balance Aβ levels. A study by Justin M. Long and his team in September 2012 demonstrates the regulatory effect of miR-153 on APP and Aβ (Amyloid-beta) production in human neurons associated with Alzheimer’s disease. Transfections with miR-153 mimics in primary human fetal brain cultures significantly decreased APP expression by approximately 20% and Aβ(1–40) levels by approximately 30%. In contrast, using an antisense inhibitor of miR-153 increased APP levels by approximately 30%, thus confirming that miR-153 suppresses APP under endogenous conditions. In addition, miR-153 has been shown to down-regulate the APLP2 protein, which has structural and functional similarities to APP, by approximately 35%. In the analyses of brain tissues of patients with Alzheimer’s disease, it was determined that miR-153 expression decreased and APP levels increased in advanced-stage disease samples. All these findings indicate that miR-153 plays an essential role in suppressing APP and Aβ production at physiological levels.122
Parkinson’s disease: dopaminergic neuron protection and repair
Parkinson’s disease is a common neurodegenerative disease in society, like Alzheimer’s.130 It affects only about 1% of adults aged 60 and over.131 Parkinson’s is a disease that begins with asymmetric bradykinesia, rigidity, and usually resting tremor.130
Previously, environmental factors were thought to be the primary risk factor for developing Parkinson’s disease. However, recent research suggests that Parkinson’s is caused by a complex interaction of genetic and environmental factors that affect many basic cellular processes.132
Parkinson’s occurs as a result of the loss of dopamine-producing cells in the substantia nigra, and this loss leads to significant impairments in neural activity and sensorimotor responses in the basal ganglia. One of the important findings in the literature is that decreased dopamine levels are associated with increased activity of GABA (γ-aminobutyric acid)-mediated inhibitory neurons in the inner segment of the globus pallidus and substantia nigra pars reticulata, which are the output regions of the basal ganglia.133
Recent studies have indicated that advanced neuroimaging techniques are used to determine diagnostic biomarkers in Parkinson’s disease. In particular, positron emission tomography, single photon emission computed tomography, and new-generation magnetic resonance imaging (MRI) methods are effective in distinguishing the disease in the early stages and from similar neurological disorders. The treatment of Parkinson’s disease is based on pharmacological approaches aimed at eliminating striatal dopamine deficiency. However, advanced treatment options such as deep brain stimulation come to the fore only in patients with permanent motor complications. Experimental treatment strategies include genetic and cellular-based applications to restore dopamine production. In addition, the intracellular accumulation and transport of α-synuclein, a protein that plays an important role in the pathogenesis of Parkinson’s disease, has been evaluated as a therapeutic target in recent years. However, one of the biggest challenges in this area is that reliable biomarkers that can define the prodromal phase of the disease before clinical symptoms appear have not yet been developed sufficiently. Identifying these markers will allow for the application of new treatment approaches that can slow down or stop the disease process at an earlier stage134 (Table 6).
Table 6.
Recent studies on the effects of miRNAs associated with Parkinson’s disease
|
miRNA
|
Disease
|
Expression change
|
Result
|
Reference
|
miR-142-3p
miR-222 |
Parkinson |
Decreased |
Serum levels of Parkinson's patients are decreased compared to healthy individuals; it has the potential to be a biomarker for early diagnosis. |
Chen et al125 |
| miR-27a |
Parkinson |
Increased |
Serum levels were found to be increased in Parkinson's patients; it can be evaluated as a biomarker for early diagnosis. |
Chen et al125 |
| miR-133b |
Parkinson |
Decreased |
This miRNA, expressed in midbrain dopaminergic neurons, was found to be deficient in Parkinson's patients; it is critical for neuron maintenance. |
Kim et al135 |
miR-34b
miR-34c |
Parkinson |
Decreased |
These miRNAs are decreased in the affected brain tissues of Parkinson's patients; it is associated with mitochondrial dysfunction, oxidative damage and DJ1/Parkin reduction. |
Miñones-Moyano et al136 |
| miR-181a |
Parkinson |
Increased |
Overexpression of miR-181a inhibited the JNK pathway and reduced neuronal apoptosis and autophagy. |
Liu et al137 |
| miR-124a |
Parkinson |
Increased |
According to its abundance in the brain and more than 40 dysregulated targets, it may play an important role in the pathogenesis of PD. |
Angelopoulouet al138 |
miR-34b
miR-34c |
Parkinson |
Decreased |
These miRNAs suppress α-synuclein expression; their decreased expression triggers α-syn increase and aggregate formation. |
Kabaria et al139 |
| miR-34b/c |
Parkinson |
Decreased |
It reduces viability in SH-SY5Y cells, impairs mitochondria, and increases oxidative stress; It reduces DJ1 and Parkin proteins. |
Miñones-Moyano et al136 |
| miR-124 |
Parkinson |
Increased |
It regulates apoptosis and autophagy by targeting bim in the MPTP model. |
Wang et al140 |
| miR-433 |
Parkinson |
Increased |
When the binding site in the FGF20 gene is disrupted, SNCA expression increases; this situation is associated with Parkinson's. |
Wang et al141 |
| miR-7 |
Parkinson |
Decreased |
It decreases in the Parkinson's model; It protects cells from oxidative stress by suppressing SNCA. |
Junn et al142 |
On February 2, 2011, Miñones-Moyano and colleagues reported that miRNAs play essential physiological and pathological roles in the central nervous system. miRNAs bind to the open reading frame and 3′ UTR regions of target miRNAs, and as a result of this binding, they destroy the miRNA or prevent protein synthesis. Due to these functions, miRNA function disorders have been associated with many neurodegenerative diseases. Although other studies have suggested that miRNAs may play a role in Parkinson’s, screening studies for a general miRNA expression disorder in the brains of Parkinson’s patients are lacking. In this study, Elena and colleagues evaluated miRNA disorders in brain regions affected to different degrees in idiopathic Parkinson’s patients at various evolutionary stages. Their aim was to reveal the disorders in gene expression networks affecting key pathogenic pathways in sporadic Parkinson’s. In this study, they showed that miR-34b/c is widely reduced in the brains of Parkinson’s patients and that this reduction starts in the early stages of the disease and affects the mechanisms underlying mitochondrial dysfunction and oxidative stress. This study used 11 patients in stages 4 and 5 and 6 healthy individuals as controls. A pool of equal amounts of total RNA taken from the mesencephalon of 11 Parkinson’s patients and 6 controls were compared, and significant differences were detected. It was shown that two miRNAs, miR-34b and miR-34c, are significantly reduced in the brain in advanced stages of Parkinson’s disease. Significant decreases were detected in the levels of these miRNAs, especially in the mesencephalon, substantia nigra, frontal cortex, and partially in the cerebellum. This decrease was seen not only in the damaged areas of Parkinson’s but also in the less affected areas. Since miR-34b and miR-34c are produced in parallel and found in different amounts in different regions of the brain, this has been thought to be a function both of region and pathological effect of the disease. It has been reasoned that the decrease in these miRNAs could be accountable for the cellular dysfunctions observed in Parkinson’s.136
Amyotrophic lateral sclerosis: motor neuron survival and inflammation control
ALS was first described as a motor neuron disease by Jean-Martin Charcot in 1869. However, today this definition is not only a motor neuron disease; it is accepted as a multisystem neurodegenerative disorder that shows disease diversity at the clinical, genetic and neuropathological levels.143
Approximately 50% of patients show extra-motor symptoms in addition to motor problems. In 10-15% of cases, an additional diagnosis of frontotemporal dementia (FTD) can be made.144 In 35-40% of patients, mild behavioral or cognitive changes can be observed. FTD is characterized by degeneration of the frontal and anterior temporal lobes and clinically presents with behavioral changes, executive function impairment, and language impairment.145
The incidence of the disease in men is 3.0 per 100 000 people per year, while this rate is 2.4 per 100 000 people per year in women. In other words, the probability of ALS disease being seen in men is higher than in women. The lifetime risk of ALS based on the general population is 1:400 for women and 1:350 for men. The onset of this disease usually occurs between the ages of 58-63. However, in patients with a family history, this age range drops to 47-52. The incidence decreases rapidly after the age of 80.146
When the clinical picture of ALS is examined, the first symptom that appears is muscle weakness, and this weakness increases with the cooling weather. This weakness is asymmetric at the beginning of the disease. While bulbar symptoms are seen in most patients in the advanced process, respiratory symptoms also develop eventually. The disease progresses insidiously, and spasticity may gradually develop in weakened atrophic limbs. This spasticity affects the patient’s dexterity and gait. In advanced stages of the disease, patients may develop flexor spasms due to excessive activation of the flexor arch in a spastic limb. In addition to the symptoms encountered, bladder dysfunction, sensory and cognitive symptoms, and multisystem involvement may sometimes be observed.147
Although it is not known for certain what causes motor neuron degeneration in ALS, it is thought that more than one cellular mechanism plays a role.148 Mutations have been found in some genes, such as SOD1, TARDBP, FUS, and C9orf72, in familial ALS cases. These mutations generally damage motor neurons because they cause harmful protein accumulation or toxic function accumulation. The accumulation of free radicals leads to cell damage and cell death. SOD1 mutations are particularly associated with this mechanism. Excessive accumulation of the excitatory neurotransmitter called glutamate causes excessive calcium entry into neurons. Excessive calcium accumulated in cells leads to mitochondrial dysfunction. Proteins that form the cytoskeleton and aggregates within the cell accumulate abnormally and disrupt cell function. In ALS, support cells such as microglia are overactivated and produce harmful inflammatory substances. This can further damage motor neurons. Levels of growth factors such as BDNF, IGF-1, and VEGF, which keep nerve cells alive, decrease in ALS, making it difficult for motor neurons to survive. In the final stage, motor neurons enter the apoptosis pathway.147
In ALS management, specialists, therapists, and palliative care nurses will all have to embrace a concerted, multidisciplinary strategy. There is no cure for the illness; its symptoms that are often experienced by such patients are treated and efforts are made to increase the quality of life of the patient.149 The reason why scientists have labored on this subject is that nobody is aware of a cure for ALS and there are many individuals in society suffering from this disease (Table S1).
Maria Liguori and colleagues conducted this study on August 28, 2018, stating that validated biomarkers for monitoring disease activity have not yet been identified, so they conducted this study to identify possible miRNA dysregulation associated with the sporadic form of the disease (sALS). 56 sALS patients and 20 healthy controls were included in the study. Peripheral blood samples of sALS patients and the control group were analyzed. As a result of these analyses, 38 miRNAs (let-7a-5p, let-7d-5p, let-7f-5p, let-7g-5p, let-7i-5p, miR-103a-3p, miR-106b-3p, miR-128-3p, miR-130a-3p, miR-130b-3p, miR-144-5p, miR-148a-3p, miR-148b-3p, miR-15a-5p, miR-15b-5p, miR-151a-5p, miR-151b, miR-16-5p, miR-182-5p, miR-183-5p, miR-186-5p, miR-22-3p, miR-221-3p, miR-223-3p, miR-23a-3p, miR-26a-5p, miR-26b-5p, miR-27b-3p, miR-28-3p, miR-30b-5p, miR-30c-5p, miR-342-3p, miR-425-5p, miR-451a, miR-532-5p, miR-550a-3p, miR-584-5p, miR-93-5p) were found to be significantly downregulated in sALS. They also found in this study that different miRNA profiles characterize bulbar or spinal onset and rate of progression. This observation supports the hypothesis that miRNAs may affect the phenotypic expression of the disease. Genes known to be associated with ALS (e.g., PARK7, C9orf72, ALS2, MATR3, SPG11, ATXN2) were also confirmed to be dysregulated in ALS patients in this study. Other potential candidate genes, such as LGALS3 (playing a role in neuroinflammation) and PRKCD (activated in mitochondrial-mediated apoptosis), were also identified in these ALS patients.156
Multiple sclerosis: immune modulation and myelin repair
Multiple sclerosis (MS) is a chronic and progressive neurodegenerative disease with autoimmune mechanisms, consisting of episodes of central nervous system inflammatory demyelination and axonal transection.157 MS typically occurs in individuals between the ages of 20 and 45, but rarely in childhood or late middle age.158 Multiple sclerosis is more common in men. MS is ever more a global phenomenon. MS prevalence increases with latitude, though this trend decreases in Norway and the United States, the sole two countries where it has been examined.159 The prevalence of MS worldwide ranges from 5 to 300 per 100 000 people and increases at higher latitudes.157 For example, there are 250 000 to 350 000 MS patients in the United States, and 50% of those who are managed require assistance walking within the next 15 years.160 The latitudinal gradient in MS prevalence can be intensely exploited by UVB exposure, which stimulates skin production of vitamin D. Low vitamin D levels, fixation of vitamin D intake, liberalization of outdoor activities, and genetic polymorphisms that cause low vitamin D levels suggest that vitamin D plays a role in the causal pathway of MS.159
In treatment, both genetics and growth rates are considered in MS. The whole thing is thought, believed to be triggered by an infectious agent; however, in many phases, something is considered autoimmune in the cellular pathogenesis of MS. The process itself inhibits the peripheral tolerance of myelin antigen-specific CD4 + T cells, thus the activation of CD4 + T cells that overcome the BBB initiates the pathophysiological events. Therein, proinflammatory effector T helper subgroups, Th17 included, are believed to play a role in the disease mechanism of organ-specific autoimmune responses.161
The first clinical symptom presents a specific central nervous system region as a clinically isolated syndrome. This condition may be limited to a single symptom or may present itself with multiple symptoms immediately. The most common initial symptoms include optic neuritis, brainstem involvement and spinal cord syndromes; However, less common initial conditions, including cortical complications, are possible. MS attacks usually develop over hours or days, reaching a plateau in a few weeks and then showing signs of deterioration. Although clinical studies in early MS often show significant remission, each relapse may leave some damage. For example, acute optic neuritis may return to normal after visual acuity; however, they do not permanently impair color perception, contrast reduction, or depth perception. In this case, the reduction in recovery limits recovery and progressive surgical loss becomes permanent.159
Disease-modifying therapies (DMTs) are effective drugs that provide the best possible life for patients with MS. DMT should be discontinued if a serious adverse event related to its treatment occurs or is suspected, especially if the side effect of the drug is life-threatening, since MS itself does not significantly increase mortality. Many MS treatments, especially the newer ones, expose patients to the risk of infectious, hematological, cardiac and neoplastic complications that are potentially fatal and require careful monitoring. Given the nature of MS, DMT discontinuation is usually temporary, but in some cases, it may be permanent. This decision is usually made according to known risk factors (e.g. the patient’s age, history of the disease, other diseases, etc). If DMT is discontinued, different drugs with a similar mechanism of action that could be used instead may worsen an adverse condition or adversely affect the recovery process. Therefore, careful consideration should be given before making a change in treatment162 (Table S2).
Rhead and colleagues investigated the contribution of miRNAs to pediatric-onset MS in their studies dated May 15, 2019. The incidence of MS in children is 5%. Epigenetic effects, including the contribution of mi-RNAs, play a significant role in the pathogenesis of adult MS. The proportion of individuals who develop MS in childhood is a higher genetic risk factor than in adults, so it was thought that mi-RNA alterations would play a greater role in childhood MS (ped-MS) than in onset MS. This success also aimed to seek evidence by obtaining miRNA contributions to the pathogenesis of ped-MS. This success has been applied with two different approaches using genetic data from a large group of ped-MS patients collected to investigate miRNAs’ role in ped-MS abroad. Firstly, in the analysis performed with the MIGWAS method, it was observed that genetic improvement was enriched in the networks where the genes targeted by miRNAs were formed, and this improvement continued, especially in tissues such as gastrointestinal, brain, and disability systems. The enrichment observed, especially in gastrointestinal tissues such as oral disease, is remarkable due to the known bidirectional relationship between MS and the discharge microbiome. Secondly, the ped-MS transmissibility of genes involved in studies provides the stability of the analysis of genetic genes (miR-SNPs) with miRNA improvement, length differences, and protein folding in the endoplasmic reticulum. In addition, some miRNAs were expressed differently only in ped-MS cases, indicating the existence of biomarkers specific to childhood MS. However, the generalizability of reproducibility may be limited by performing the predicted miRNA-target operations and limiting the analyses to white individuals only. Nevertheless, this study provides important data on the genetic structure of ped-MS that miRNAs can be effective in and suggests strong candidate genes, pathways, and miRNAs to store them.169
Spinal cord injury: axon regeneration and functional recovery
SCI is a neurological condition that affects 250,000-500 000 people each year, with an estimated 2 to 3 million people worldwide living with a disability related to SCI, disrupting the patient’s entire life.170 This neurological condition is a devastating and widespread disorder that has profound physical, psychosocial, and socioeconomic impacts on modern society.171 The demographics of SCIs are increasingly affected as individuals age.172 The incidence is 2-5 times higher in men and peaks in younger adults. Patients with SCI are 2-5 times more likely to die at an early age and have even worse survival rates in low- and middle-income countries.170
The most common causes of SCI were automobile accidents (31.5%) and falls (25.3%), followed by gunshot wounds (10.4%), motorcycle accidents (6.8%), diving accidents (4.7%), and medical/surgical complications (4.3%). Collectively, these account for 83.1% of total SCIs since 2005. Automobile accidents were the leading cause of SCI up to age 45, while falls were the leading cause after age 45. Injury-causing factors changed: before 45 years of age, mostly automobiles caused SCIs, while some other factors like falls became predominantly responsible after 45 years of age. The rate of injuries due to a gunshot wound showed the starkest variation when it came to race/ethnicity. More accident cases of SCIs took place on the weekend and in the warmer months. This is in parallel with peaks in SCIs due to motorcycle and diving accidents.173
From a pathophysiological perspective, the initial mechanical trauma (primary injury) causes an increase in the permeability of neurons and glial cells. This triggers a secondary injury process that leads to progressive cell death over the following days and weeks. Over time, remodeling occurs at the lesion site; the resulting cystic spaces and glial scar tissue seriously impede nerve regeneration.172 At the same time, SCI negatively impacts the patient’s life in many ways. Impaired bowel and bladder function, sexual dysfunction, motility, and autonomic functions, along with secondary conditions such as pressure ulcers and pain, are just a few of the consequences that can directly affect a person’s health.174
Diagnosing SCI requires a comprehensive patient history, standard neurological physical examination, and radiographic imaging of the spinal cord. After diagnosis, various interventions must be implemented rapidly, including hemodynamic monitoring in the intensive care unit, early surgical decompression, blood pressure elevation, and potentially methylprednisolone administration.172
In the early stages of SCI, axonal regeneration in the damaged areas and the re-formation of axo-glial triple junctions are the basic conditions for an effective repair process. The transplantation of stem cells that can form neuronal and glial cell lines to these areas has positively contributed to the recovery process after SCI. Numerous preclinical studies using various stem or progenitor cell types have shown promising results, especially in the acute and subacute periods. However, a significant decrease in these recovery rates is observed when the chronic stages of injury are reached. Therefore, developing new treatment approaches that will reduce the effect of secondary complications and improve the quality of life of individuals with spinal cord injuries stands out as a critical need today175 (Table S3).
This is a description of a 2009 study by Liu et al, in which 350 miRNAs were analyzed after acute SCI in rats. Of 269 miRNAs expressed in the spinal cord, 97 underwent significant change after SCI. The miRNAs were divided into five classes: which were increased continuously in expression, decreased, or increased and then decreased in expression. Amongst the increases of miRNAs that suppress anti-inflammatory genes, such as miR-21, the decreases suppress inflammatory genes such as miR-181a and miR-137. Some of the miRNAs that increase after SCI inhibit neurons by targeting antioxidant (e.g., SOD1, catalase) and antiapoptotic (e.g., Bcl2) genes. The microarray results, when compared with those of RT-PCR, were found to be reliable. These findings revealed the importance of miRNAs in inflammation, oxidative stress, and apoptosis after SCI. At the same time, some miRNAs suppress proinflammatory and proapoptotic genes after SCI, while others suppress anti-inflammatory, antioxidant, and antiapoptotic genes, thus inhibiting healing. This suggests that miRNAs contribute to the development of secondary damage after SCI. In other words, this study demonstrates that miRNAs have potential as a therapeutic target.182
Huntington’s disease
HD is an autosomal dominant, progressive neurodegenerative disease with a distinctive phenotype including chore and dystonia, incoordination, cognitive decline, and behavioral difficulties.183 HD is an autosomal dominantly inherited disease.184 The prevalence of HD varies worldwide. In Western and European populations, it is generally reported to be around 4–10 per 100 000 people; however, a more recent study from the United Kingdom suggests it may be closer to 12.3 per 100 000 population. These rates make HD one of the most common inherited neurological diseases.185 HD usually affects patients between the ages of 30 and 50. However, the longer the CAG repeats, the earlier the symptoms begin.186
HD is associated with a toxic gain-of-function and/or loss of normal HTT function of the mutant HTT (mHTT) protein resulting from mutations in the HTT gene.187 A lengthened CAG triplet results in HD repeat within exon 1 of the huntingtin protein gene on the short arm of chromosome 4. It results in a stretched polyglutamine (PolyQ) repeat at the N-terminus of the causative protein.185 The disease is caused by extensive degeneration of GABAergic medium spiny neurons, especially in the striatum (95%). Simultaneously, other regions of the brain such as the cortex, globus pallidus, cerebellum, amygdala, and hippocampus may be affected. Various mechanisms are involved in its pathogenesis: intracytoplasmic and intranuclear inclusions formed due to the accumulation of mHTT may disrupt the ubiquitin-proteasome system; transcriptional dysregulation and glutamate excitotoxicity may cause neuronal damage. In addition to this, mitochondrial dysfunction, impaired energy metabolism, neuroinflammation, axonal transport, and dysregulation of synaptic transmission also contribute to the pathogenesis. The age of onset of the illness may be determined by genetic modifiers.187 Motor disorder seen in HD usually begins with involuntary choreic movements of the distal limbs and progresses to the proximal muscles later; hypokinetic features such as bradykinesia, rigidity, and dystonia become prominent in the later stages of the disease. Patients gradually experience gait disorders, dysarthria, dysphagia, muscle contractures, and balance loss, and in advanced stages, they become entirely dependent on care. Psychiatric symptoms often begin before the motor symptoms of the disease and include findings consistent with frontal lobe dysfunction, such as impulsivity, irritability, depression, apathy, and loss of insight. Cognitive impairment begins early, is particularly evident in executive functions, and may progress to subcortical dementia over time. Other possible symptoms include cerebellar ataxia, oculomotor apraxia, eye movement disorders, and seizures in patients with juvenile-onset. The clinical course is typically examined in three stages: prodromal, presymptomatic, and symptomatic; the disease diagnosis can be confirmed by genetic testing, and treatment planning is made according to the severity of symptoms. The average survival time is 15–18 years, and symptoms may be more benign in late-onset cases187 (Table S4).
By targeted molecular genetic analysis, HD is diagnosed by detecting CAG trinucleotide repeats in the HTT gene in individuals with clinical signs and symptoms. Pathogenic CAG repeat expansions that cause HD cannot be detected by current clinical array-based multigene panels, exome, or genome sequencing; therefore, targeted analysis methods that specifically measure the number of CAG repeats should be used for diagnosis. Alleles are classified according to the number of CAG repeats. Those with up to 26 repeats are considered normal, and those between 27 and 35 are considered to be in the intermediate range, which may carry a disease risk to future generations, even if they do not exhibit symptoms. Repeats of 36 and above are pathogenic for HD; alleles between 36 and 39 have reduced penetrance, and symptoms may not develop continuously, while repeats of 40 and above have complete penetrance. HD development is almost certain within the individual’s lifetime. The accuracy of genetic diagnosis is 100%, and genetic counseling plays a crucial role in evaluating individuals at risk188 (Table S5).
Challenges and considerations in clinical translation
Stability and scalability of exosome production
Exosomes are EVs that provide communication between cells and have the potential for diagnostic and therapeutic use thanks to the biomolecules they carry.193 However, the fact that these exosomes can only be produced safely in a laboratory environment does not mean they will be suitable and reliable for clinical use. Therefore, exosome production stages should be efficient regarding both stability and scalability. These two factors are the most important factors that directly affect the success to be achieved in clinical applications.153 Most current methods used to produce exosomes today are suitable for small-volume production, so the methods used today do not provide the quantity and quality required for clinical application.194
Exosomes are a cell-free therapeutic modality with potential but cannot be stored for long periods of time. Therefore, it is necessary to analyze the preservation technology of exosomes to keep their biological activities, deliver them, and make them useful for clinical practice. The methods used for the preservation of exosomes are freezing, freeze-drying, and spray-drying.195
Although exosomes have great potential as drug delivery systems, their transformation into pharmaceutical products is limited due to stability issues.196
However, if exosomes are to be used as drug delivery vehicles, they must be stable.196 In addition, pre-isolation, isolation processes, and subsequent formulations must also be stable. For exosomes, stability can be defined as the resistance developed against aggregation, structural degradation, and protein degradation. Therefore, more than one method should be used to assess exosome stability. Measuring the levels of surface markers CD63 or CD81 can be used to understand whether protein degradation has occurred. For drug delivery applications, the colloidal stability of exosomes is essential, and it is assessed by zeta potential, size distribution, and concentration measurements. Nanoparticle tracking analysis (NTA) is recommended to measure these parameters. Electron microscopy is the only technique that can physically see the morphology of exosomes, but since it depends on the operator, the results may not always reflect the correct results.197 The main factors affecting stability are temperature, storage time, buffer solutions used, freeze-thaw cycles, and cryoprotectants. Most studies have examined the effect of temperature and have found that -70 °C to -80 °C are generally optimal, but it is stated that these temperatures will be problematic for practical applications. Alternative techniques, such as lyophilization, have been stated to have the potential to increase stability, especially for cargo-carrying exosomes. However, long-term and detailed stability data on drug-loaded exosomes are still limited196 (Table S6).
In order to switch to clinical use of exosomes with these applications, obtaining pure, functional, and stable exosomes is a fundamental requirement, and the scalability of this production process is essential. Traditionally, ultracentrifugation (UC) is unsuitable for industrial production due to low yield, long processing time, and reproducibility problems.204 Although UC is sufficient for limited amounts of exosomes obtained from small-volume cell cultures, it is insufficient in cases where the gram-level product is required for clinical applications.205 Large doses of EVs are required to achieve a significant clinical effect, which significantly exceed the yield of natural vesicles secreted by cells, and therefore, UC remains an inadequate method. Therefore, a robust, cost-effective, and scalable method is required to produce clinical-quality EVs. Each of the large-scale EV production methods has its advantages and disadvantages. The first of these methods, bioreactor culture, is important in producing EVs obtained from cells. 2D systems (multilayer and bottle systems) offer GMP-compliant manufacturing options but are generally limited by low efficiency. 3D systems (hollow fiber bioreactors and microcarriers) offer higher cell density and EV yield, and hollow fiber bioreactors can further increase the yield by a factor of 40 by achieving maximum surface area. However, biological limitations such as cell aging, spontaneous differentiation, and culture homogeneity in bioreactor systems should be considered. Another method applied for EV production is cell stimulation; this method can cause cells to produce more EVs with factors such as serum starvation, pH, temperature, and hypoxia. In addition, chemical (DTT, formaldehyde, calcium ionophores, etc) and physical (shear stress, acoustic treatment, etc) stimuli will effectively increase EV release. In induced EV production, these methods offer the advantage of obtaining high yields and more efficient production opportunities when combined with bioreactor systems. The biomimetic vesicle production method allows EV-like structures to be obtained with physical disintegration methods such as ultrasound homogenization and nitrogen cavitation. It produces EVs with homogeneous size, high yield, and homogeneous composition by requiring low labor and low time cost. However, the quality control of these methods is of great importance; controls such as morphology, homogeneity, biological activity tests, toxicity, and immunogenicity tests play a critical role in obtaining safe and effective products. Although UC is generally used for EV isolation, this method carries the risk of low yield and contamination with medium proteins. As a result of this, more effective methods such as microfiltration, size exclusion chromatography, and immunization are gaining more popularity today. Overall, the combination of different EV production methods and considering the advantages of each has implications for the effectiveness and safety of EV production on a large scale, and the optimization of the methods will pave the way for bringing new EV-based therapies into clinical practice.194
Standardization of isolation and purification techniques
Stem cell-derived miRNA-loaded exosomes hold potential as biological carriers in neurological regeneration therapy.206 However, to become consistent and applicable for clinical use is dependent on isolating and purifying procedures being standardized. There are limitations in preserving exosome biological activity and purity207 Therefore, for clinical application, miRNA-loaded exosomes manufacturing requires standardized isolation and purification methods with minimum efficiency loss, purity, and preservation of biological function.206
Various methods of isolation are used to isolate stem cell-derived exosomes. These include differential UC, density gradient centrifugation, ultrafiltration, pre-entrapment (PEG), and flow cytometry. Each has its advantages and disadvantages208-211 (Table S7).
The morphology of the obtained EVs is also affected by the methods used in sample preparation and characterization after isolation. Defining EV subtypes precisely is important for determining characterization and isolation methods. One of the difficulties in using exosomes in the clinic is the lack of characterization standards. The aim of the characterization stage after exosome isolation is to have a certain level of purity and yield. This starts with the quantification of proteins, lipids, and nucleic acids. Additional procedures are required if the sample does not meet the desired purity and yield. Then, appropriate methods should be selected for bulk and single vesicle characterization to complete the characterization process. In other words, high-purity samples and usually more than one orthogonal method are required to characterize exosomes accurately. Some traditional methods have been used to achieve this. The first of these methods is NTA. NTA is a technique used to measure the sizes and concentrations of particles in exosome samples. However, NTA does not provide high accuracy in inhomogeneous samples and has difficulty correctly analyzing tiny particles. Another technique is dynamic light scattering (DLS). DLS uses the principle of light scattering to measure the size of exosomes. However, DLS cannot accurately reflect the size distribution and sample heterogeneity can affect DLS measurements. Another method is flow cytometry. Flow cytometry is a technique used to measure exosome size and surface properties. However, since exosomes are generally limited to 30–150 nm, this device cannot accurately distinguish small particles of this size. The last frequently used method is electron microscopy. Electron microscopy is used to observe exosome morphology, structure, and topology. This method usually requires fixation and drying of the sample, which can affect the morphological structure of exosomes. In other words, when all these methods are considered classic, they still require repetitions to obtain accurate results. At the same time, some methods applied in these techniques change the content of the sample, thus affecting the results’ reliability.212
Exosome isolation and purification still pose significant problems for the studies that were carried out. One of the biggest reasons for this problem is the complex and unstable structure of the exosome. There is still no consensus in the literature on which purification method is the best, and disagreements on this issue continue. At the same time, co-isolation of other vesicles and non-vesicular molecules occurs, which prevents data comparison between research laboratories. However, the main goal when performing isolation and purification is for an ideal isolation method to be fast, reproducible, easy to perform, flexible, and not require special equipment.213
Targeted delivery and crossing the BBB
Delivering drugs to the brain is tricky due to the BBB between the blood and the brain. The BBB, which consists of tight junctions between cells, is a functional and vital barrier because it prevents harmful substances from passing into the brain. However, in order to deliver drugs, it is necessary to pass the BBB, and to do this, it is necessary to deliver the drug with carriers that can pass the BBB because cell transplantation, which has been tried as an alternative to this transportation difficulty, has not been able to achieve high efficiency in treatment.214,215
Among these carriers, exosome stands out as an important potential carrier.216 Because exosomes can largely cross the BBB, have high bioavailability, have natural targeting ability, can use intracellular transport routes, and have high stability in the body. Since they are accepted naturally by the body due to their origin in the cell, their immunogenicity is also relatively low.217-219 Exosomes can cross the BBB and the blood-cerebrospinal fluid barrier, the blood-retinal barrier, and many other biological barriers that many drugs have difficulty passing. There are various accepted models on how exosomes achieve this, such as the ability of exosomes to pass through/between tight junctions, but no definitive information is yet available.218 Drug delivery with exosomes will reach perfection if the challenges related to using exosomes, such as producing sufficient amounts of pure exosomes, drug loading efficiency, controlled targeting and distribution, and industrial production scale, are resolved.217
Immunogenicity and safety concerns
Interest in exosomes is increasing day by day. An article prepared in 2022 stated that there were 116 clinical studies on exosomes at that time.220 Today, this number has reached 273.221 The potential of exosomes to be used as intercellular molecule carriers is undeniable. Due to their natural cellular structure, exosomes resist phagocytosis or destruction by macrophages. In addition, it is a significant advantage that they do not trigger the immune system. However, the fact that the behavior of exosomes in the body is not yet fully understood shows that questions about these molecules are still being answered.222 However, it is known that exosomes have high biocompatibility and therefore have a low risk of side effects. Difficulties in using exosomes mainly arise from technological and logistical problems.223
There are studies comparing exosomes with alternative carriers. For example, when lipid nanoparticles were compared with exosomes for mRNA transport, it was found that exosomes did not show any toxic effects at any dose, both in vitro and in vivo. Cellular toxicity was observed in lipid nanoparticles. In addition, mRNAs carried through exosomes created a strong and permanent immune response, both cellular and humoral.224 One of the obstacles to the practical use of exosomes is that standardization regarding clinical application has not yet been achieved. There is currently no clarity on which disease, when, and what dose of exosomes will be applied. This situation hinders the safe use of exosomes.225 However, solutions to these problems will be found as studies on exosomes deepen.
Future perspectives and emerging trends
miRNAs and exosomes play essential roles in neural repair mechanisms and are increasingly recognized for their therapeutic potential in neuroregenerative medicine. Emerging research trends underscore the advancement of personalized exosome therapies, wherein patient-derived vesicles are bioengineered to enable targeted neuromodulation within the nervous system. Simultaneously, CRISPR-based bioengineering strategies are being employed to enhance the specificity and efficacy of miRNA delivery across neural tissues. Moreover, combination approaches that integrate exosomes with biomaterials or small-molecule compounds are being explored to achieve synergistic neuroregenerative effects. In parallel, significant progress in in vivo imaging and tracking technologies is facilitating real-time visualization of exosome biodistribution and dynamic function, thus enabling more precise monitoring and optimization of therapeutic efficacy (Figure 4).
Figure 4.
A schematic overview of the potential future applications and emerging research trends of miRNAs and exosomes in neuroregeneration
.
A schematic overview of the potential future applications and emerging research trends of miRNAs and exosomes in neuroregeneration
Personalized exosome therapy in neurology
Personalized medicine means selecting the most appropriate treatment methods for individuals based on their genetic and pharmacogenomic characteristics. As medical technologies develop, molecular diagnosis and treatment methods will be more compatible with clinical use, and personalized medicine, which is more efficient than the trial-and-error method, will find more space for itself.226
Biomarkers and integrated diagnosis and treatment methods are essential in diagnosing and treating neurological diseases. Especially considering the brain has a limited capacity to repair itself, interest in regenerative treatments has increased.227 In this context, personalized medicine studies on neurological diseases are supported by a wide variety of genetic studies such as neurogenomics, neuroproteomics, neurometabolomics, and non-genetic studies such as cell/gene therapy and nanobiotechnology.228
Exosomes, the ideal blend of the qualities desirable for personalized medicine, such as high sensitivity and effective delivery to the target location, are versatile tools that perform vital roles in physiological and pathological processes. Exosomes, according to existing evidence, will hold a large niche in disease diagnosis and treatment in the age of personalized medicine.229 This is a critical process in managing progressive neurodegenerative diseases such as Alzheimer’s, where early diagnosis is required.230 Studies on exosomes, such as this one, need to be carried out to an internationally acceptable standard and come up with detection of exosomes.231
One of the conditions that make treatment difficult in neurological diseases such as Alzheimer’s, Parkinson’s, ALS, MS, stroke, brain tumors, and psychiatric diseases is that traditional drugs cannot cross the BBB. Exosomes, one of the systems being studied to solve this problem, can successfully deliver the contents they carry to their targets thanks to their biocompatibility, not triggering an immune response, and their ability to pass the BBB naturally.232 In this way, many drugs with low bioavailability due to difficulty passing the BBB can show their effects more successfully and more safely.233 Exosomes give quite satisfactory results when used alone and as developed systems such as luteolin/mannose-pEXO.234
CRISPR and bioengineering approaches for optimized miRNA delivery
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-Cas is a natural defense system bacteria develop against viruses. Scientists have transformed this system into a tool that can make pinpoint changes to edit genetic material, and it has become a technology that can be used in almost every field. CRISPR technologies have a wide range of applications and great potential in neuroscience, one of their areas of use.235 Considering that diagnosing diseases correctly and on time is closely related to success in treatment, it is seen that CRISPR technologies have potential in diagnosis and treatment.236
Like every promising system, CRISPR technologies also have aspects that need to be improved. Increased specificity is one of them. As the specificity of CRISPR technologies increases, unwanted extracellular effects decrease, and therapeutic agents are delivered only to the cells they need to go to when they need to go. This effect can be achieved with CRISPR MiRAGE. CRISPR MiRAGE recognizes miRNA-Argonaute complexes in the target cell via single-guide RNA (sgRNA) and decides whether the CRISPR system should be active or not. CRISPR MiRAGE has been tested on the Duchenne Muscular Dystrophy (DMD) model and has yielded successful results. Thus, safer, more effective, and more specific treatments have been paved with CRISPR MiRAGE.237
miRNAs and the CRISPR system have mutual positive effects on each other. Tools such as the CRISPR/dCas9 system, which can bind to specific gene regions without cutting DNA and increase gene expression, open new horizons in translational medicine by directing target miRNAs to the gene region with the help of guide RNA and regulating gene expression.238
Combination therapies: exosomes with biomaterials and small molecules
In severe and life-threatening conditions such as spinal cord injuries, the application of a single treatment method is often insufficient. Studies show that combination treatments, in which more than one method is used in combination, are more effective than a single method in many disorders, including SCI.239,240 In disorders such as MS, where one of the main obstacles to treatment is the BBB, exosomes can be loaded with various materials more safely in terms of toxicity compared to similarly purposed nanoparticles and can reach the target without being recognized by the immune system.241,242 However, this therapeutic potential of exosomes has been reflected in clinical applications to a limited extent due to the difficulties in directly delivering them to the target tissue and controlling their release. To overcome these difficulties, the combined use of exosomes with various medical tools such as biomaterials, carrier systems, and controlled-release implants is one of the effective solution options.243
Advances in in vivo imaging and tracking of exosome dynamics
The natural properties of EVs, of which exosomes are a member, are quite suitable for them to be drug carriers. However, for EVs to be drug carriers, it is necessary to know how they are distributed in the body, which organs they go to, and how long they remain. To obtain this information, following EVs in vivo animal models is very important. Because the vast majority of the information obtained so far has come from the in vitro environment. Since each imaging technique has its advantages and disadvantages, the technique to be used should be selected according to the type of data needed and the target.244,245
For example, bioluminescence (BLI) imaging, an optical imaging technology, is the most widely used in vivo molecular tracking method, especially for animal studies. BLI is just a matter of providing a unique genetic marker (e.g., luciferase enzyme) to viable cells initially, with later expression of this enzyme in the cells, and then detection of luminescence with its substrate (e.g., luciferin).246 Within the zebrafish embryo system, the CD63-pHluorin green fluorescent protein used for imaging has been supplemented with proteome analysis to trace EVs in high spatial and temporal resolution, and thus determine the functional role of exosomes in interorgan communication.247 Similarly, the Cre-LoxP genetic labeling system has been successful in tracing EVs secreted from tumor cells in vivo.248 It has also been reported that more advanced monitoring can be done with the use of fluorescent and bioluminescent reporters together.249
In addition to optical methods, exosome tracking can be done with methods such as MRI.250 While exosomes become visible with MRI, it is an important advantage that their structural and physiological integrity is preserved.251
Conclusion
In recent years, researchers have become increasingly interested in the ability of stem cell-derived exosomes to carry miRNAs that influence brain repair. Unlike synthetic drug carriers, exosomes can cross biological barriers naturally and interact with recipient cells without triggering a strong immune response. This unique feature has paved the way for exploring them as vehicles for the delivery of therapeutic RNA molecules for neurodegenerative disease treatment. Several independent studies have shown that exosomes loaded with certain miRNAs have the capacity to decrease inflammation, promote neuronal growth, and even promote survival of damaged neurons. For example, in animal models of Alzheimer’s and Parkinson’s diseases, such exosomal treatments have led to dramatic memory and mobility improvements. For all these advances, there are still key questions to be resolved. Among them is the determination of reproducible methods of producing high-quality exosomes at larger quantities. Another is to improve how we target them to specific areas of the brain. Yet, with growing advances in both biotechnology and molecular neuroscience, these hurdles may soon be overcome. If so, miRNA-based exosome therapies could play an important role in future treatment strategies. Currently intractable neurological conditions.
Competing Interests
The authors declare no conflicts of interest.
Ethical Approval
Not applicable.
Intelligence Use Disclosure
This article has not utilized artificial intelligence (AI) tools for research and manuscript development, as per the GAMER reporting guideline.
Supplementary Files
Supplementary file 1 contains Table S1-S7.
(pdf)
References
- Nazimek K, Bryniarski K, Santocki M, Ptak W. Exosomes as mediators of intercellular communication: clinical implications. Pol Arch Med Wewn 2015; 125(5):370-80. doi: 10.20452/pamw.2840 [Crossref] [ Google Scholar]
- Han C, Sun X, Liu L, Jiang H, Shen Y, Xu X. Exosomes and their therapeutic potentials of stem cells. Stem Cells Int 2016; 2016:7653489. doi: 10.1155/2016/7653489 [Crossref] [ Google Scholar]
- Tan F, Li X, Wang Z, Li J, Shahzad K, Zheng J. Clinical applications of stem cell-derived exosomes. Signal Transduct Target Ther 2024; 9(1):17. doi: 10.1038/s41392-023-01704-0 [Crossref] [ Google Scholar]
- Burke J, Kolhe R, Hunter M, Isales C, Hamrick M, Fulzele S. Stem cell-derived exosomes: a potential alternative therapeutic agent in orthopaedics. Stem Cells Int 2016; 2016:5802529. doi: 10.1155/2016/5802529 [Crossref] [ Google Scholar]
- Adlakha YK, Seth P. The expanding horizon of microRNAs in cellular reprogramming. Prog Neurobiol 2017; 148:21-39. doi: 10.1016/j.pneurobio.2016.11.003 [Crossref] [ Google Scholar]
- Tang X, Sun C. The roles of microRNAs in neural regenerative medicine. Exp Neurol 2020; 332:113394. doi: 10.1016/j.expneurol.2020.113394 [Crossref] [ Google Scholar]
- Dasgupta I, Chatterjee A. Recent advances in miRNA delivery systems. Methods Protoc 2021; 4(1):10. doi: 10.3390/mps4010010 [Crossref] [ Google Scholar]
- Yang N. An overview of viral and nonviral delivery systems for microRNA. Int J Pharm Investig 2015; 5(4):179-81. doi: 10.4103/2230-973x.167646 [Crossref] [ Google Scholar]
- Zhou S, Ding F, Gu X. Non-coding RNAs as emerging regulators of neural injury responses and regeneration. Neurosci Bull 2016; 32(3):253-64. doi: 10.1007/s12264-016-0028-7 [Crossref] [ Google Scholar]
- Chen Y, Gao DY, Huang L. In vivo delivery of miRNAs for cancer therapy: challenges and strategies. Adv Drug Deliv Rev 2015; 81:128-41. doi: 10.1016/j.addr.2014.05.009 [Crossref] [ Google Scholar]
- Wang H, Jiang Y, Peng H, Chen Y, Zhu P, Huang Y. Recent progress in microRNA delivery for cancer therapy by non-viral synthetic vectors. Adv Drug Deliv Rev 2015; 81:142-60. doi: 10.1016/j.addr.2014.10.031 [Crossref] [ Google Scholar]
- Nayerossadat N, Maedeh T, Ali PA. Viral and nonviral delivery systems for gene delivery. Adv Biomed Res 2012; 1:27. doi: 10.4103/2277-9175.98152 [Crossref] [ Google Scholar]
- Zhang Y, Wang Z, Gemeinhart RA. Progress in microRNA delivery. J Control Release 2013; 172(3):962-74. doi: 10.1016/j.jconrel.2013.09.015 [Crossref] [ Google Scholar]
- Zhang Y, Liu Q, Zhang X, Huang H, Tang S, Chai Y. Recent advances in exosome-mediated nucleic acid delivery for cancer therapy. J Nanobiotechnology 2022; 20(1):279. doi: 10.1186/s12951-022-01472-z [Crossref] [ Google Scholar]
- Shen Y, Cai J. The importance of using exosome-loaded miRNA for the treatment of spinal cord injury. Mol Neurobiol 2023; 60(2):447-59. doi: 10.1007/s12035-022-03088-8 [Crossref] [ Google Scholar]
- Elsharkasy OM, Nordin JZ, Hagey DW, de Jong OG, Schiffelers RM, El Andaloussi S. Extracellular vesicles as drug delivery systems: why and how?. Adv Drug Deliv Rev 2020; 159:332-43. doi: 10.1016/j.addr.2020.04.004 [Crossref] [ Google Scholar]
- Saka OM, Dora DD, Kibar G, Tevlek A. Expanding the role of exosomes in drug, biomolecule, and nanoparticle delivery. Life Sci 2025; 368:123499. doi: 10.1016/j.lfs.2025.123499 [Crossref] [ Google Scholar]
- Ferreira D, Moreira JN, Rodrigues LR. New advances in exosome-based targeted drug delivery systems. Crit Rev Oncol Hematol 2022; 172:103628. doi: 10.1016/j.critrevonc.2022.103628 [Crossref] [ Google Scholar]
- Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev 2016; 106(Pt A):148-56. doi: 10.1016/j.addr.2016.02.006 [Crossref] [ Google Scholar]
- Fang Z, Zhang X, Huang H, Wu J. Exosome based miRNA delivery strategy for disease treatment. Chin Chem Lett 2022; 33(4):1693-704. doi: 10.1016/j.cclet.2021.11.050 [Crossref] [ Google Scholar]
- Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci 2018; 75(2):193-208. doi: 10.1007/s00018-017-2595-9 [Crossref] [ Google Scholar]
- Huotari J, Helenius A. Endosome maturation. EMBO J 2011; 30(17):3481-500. doi: 10.1038/emboj.2011.286 [Crossref] [ Google Scholar]
- Schmidt O, Teis D. The ESCRT machinery. Curr Biol 2012; 22(4):R116-20. doi: 10.1016/j.cub.2012.01.028 [Crossref] [ Google Scholar]
- Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell 2005; 122(5):735-49. doi: 10.1016/j.cell.2005.06.043 [Crossref] [ Google Scholar]
- Essandoh K, Fan GC. Insights into the mechanism of exosome formation and secretion. In: Tang Y, Dawn B, eds. Mesenchymal Stem Cell Derived Exosomes. Boston: Academic Press; 2015. p. 1-19. doi: 10.1016/b978-0-12-800164-6.00001-0.
-
Jangam TC, Desai SA, Patel VP, Pagare NB, Raut ND. Exosomes as therapeutic and diagnostic tools: advances, challenges, and future directions. Cell Biochem Biophys. 2025. doi: 10.1007/s12013-025-01730-5.
- Record M, Carayon K, Poirot M, Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta 2014; 1841(1):108-20. doi: 10.1016/j.bbalip.2013.10.004 [Crossref] [ Google Scholar]
- Abramowicz A, Widlak P, Pietrowska M. Proteomic analysis of exosomal cargo: the challenge of high purity vesicle isolation. Mol Biosyst 2016; 12(5):1407-19. doi: 10.1039/c6mb00082g [Crossref] [ Google Scholar]
- Li SP, Lin ZX, Jiang XY, Yu XY. Exosomal cargo-loading and synthetic exosome-mimics as potential therapeutic tools. Acta Pharmacol Sin 2018; 39(4):542-51. doi: 10.1038/aps.2017.178 [Crossref] [ Google Scholar]
- Ferguson SW, Nguyen J. Exosomes as therapeutics: the implications of molecular composition and exosomal heterogeneity. J Control Release 2016; 228:179-90. doi: 10.1016/j.jconrel.2016.02.037 [Crossref] [ Google Scholar]
- Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K. ExoCarta: a web-based compendium of exosomal cargo. J Mol Biol 2016; 428(4):688-92. doi: 10.1016/j.jmb.2015.09.019 [Crossref] [ Google Scholar]
- Simpson RJ, Kalra H, Mathivanan S. ExoCarta as a resource for exosomal research. J Extracell Vesicles 2012; 1:18374. doi: 10.3402/jev.v1i0.18374 [Crossref] [ Google Scholar]
- Nachmias D, Frohn BP, Sachse C, Mizrahi I, Elia N. ESCRTs - a multi-purpose membrane remodeling device encoded in all life forms. Trends Microbiol 2025; 33(6):665-87. doi: 10.1016/j.tim.2025.01.009 [Crossref] [ Google Scholar]
- Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 2001; 106(2):145-55. doi: 10.1016/s0092-8674(01)00434-2 [Crossref] [ Google Scholar]
- Hurley JH. ESCRTs are everywhere. EMBO J 2015; 34(19):2398-407. doi: 10.15252/embj.201592484 [Crossref] [ Google Scholar]
- Babst M. MVB vesicle formation: ESCRT-dependent, ESCRT-independent and everything in between. Curr Opin Cell Biol 2011; 23(4):452-7. doi: 10.1016/j.ceb.2011.04.008 [Crossref] [ Google Scholar]
- Henne WM, Stenmark H, Emr SD. Molecular mechanisms of the membrane sculpting ESCRT pathway. Cold Spring Harb Perspect Biol 2013; 5(9):a016766. doi: 10.1101/cshperspect.a016766 [Crossref] [ Google Scholar]
- Hierro A, Sun J, Rusnak AS, Kim J, Prag G, Emr SD. Structure of the ESCRT-II endosomal trafficking complex. Nature 2004; 431(7005):221-5. doi: 10.1038/nature02914 [Crossref] [ Google Scholar]
- Obita T, Saksena S, Ghazi-Tabatabai S, Gill DJ, Perisic O, Emr SD. Structural basis for selective recognition of ESCRT-III by the AAA ATPase Vps4. Nature 2007; 449(7163):735-9. doi: 10.1038/nature06171 [Crossref] [ Google Scholar]
- Vietri M, Radulovic M, Stenmark H. The many functions of ESCRTs. Nat Rev Mol Cell Biol 2020; 21(1):25-42. doi: 10.1038/s41580-019-0177-4 [Crossref] [ Google Scholar]
- Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci 2013; 126(Pt 24):5553-65. doi: 10.1242/jcs.128868 [Crossref] [ Google Scholar]
- Wang X, Han S, Liang J, Xu C, Cao R, Liu S. Essential role of Alix in regulating cardiomyocyte exosome biogenesis under physiological and stress conditions. J Mol Cell Cardiol 2024; 190:35-47. doi: 10.1016/j.yjmcc.2024.04.001 [Crossref] [ Google Scholar]
- Stuffers S, Sem Wegner C, Stenmark H, Brech A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 2009; 10(7):925-37. doi: 10.1111/j.1600-0854.2009.00920.x [Crossref] [ Google Scholar]
- Zhu H, Guariglia S, Yu RY, Li W, Brancho D, Peinado H. Mutation of SIMPLE in Charcot-Marie-Tooth 1C alters production of exosomes. Mol Biol Cell 2013; 24(11):1619-37. doi: 10.1091/mbc.E12-07-0544 [Crossref] [ Google Scholar]
- Kenific CM, Zhang H, Lyden D. An exosome pathway without an ESCRT. Cell Res 2021; 31(2):105-6. doi: 10.1038/s41422-020-00418-0 [Crossref] [ Google Scholar]
- Wei D, Zhan W, Gao Y, Huang L, Gong R, Wang W. RAB31 marks and controls an ESCRT-independent exosome pathway. Cell Res 2021; 31(2):157-77. doi: 10.1038/s41422-020-00409-1 [Crossref] [ Google Scholar]
- Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F, Pérez-Hernández D, Vázquez J, Martin-Cofreces N. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun 2013; 4:2980. doi: 10.1038/ncomms3980 [Crossref] [ Google Scholar]
- Garcia-Martin R, Wang G, Brandão BB, Zanotto TM, Shah S, Kumar Patel S. MicroRNA sequence codes for small extracellular vesicle release and cellular retention. Nature 2022; 601(7893):446-51. doi: 10.1038/s41586-021-04234-3 [Crossref] [ Google Scholar]
- Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008; 319(5867):1244-7. doi: 10.1126/science.1153124 [Crossref] [ Google Scholar]
- Dennis JW, Lau KS, Demetriou M, Nabi IR. Adaptive regulation at the cell surface by N-glycosylation. Traffic 2009; 10(11):1569-78. doi: 10.1111/j.1600-0854.2009.00981.x [Crossref] [ Google Scholar]
- Jiang H, Zhao H, Zhang M, He Y, Li X, Xu Y. Hypoxia induced changes of exosome cargo and subsequent biological effects. Front Immunol 2022; 13:824188. doi: 10.3389/fimmu.2022.824188 [Crossref] [ Google Scholar]
- Wang B, Yao K, Huuskes BM, Shen HH, Zhuang J, Godson C. Mesenchymal stem cells deliver exogenous microRNA-let7c via exosomes to attenuate renal fibrosis. Mol Ther 2016; 24(7):1290-301. doi: 10.1038/mt.2016.90 [Crossref] [ Google Scholar]
- Pomatto MAC, Bussolati B, D’Antico S, Ghiotto S, Tetta C, Brizzi MF. Improved loading of plasma-derived extracellular vesicles to encapsulate antitumor miRNAs. Mol Ther Methods Clin Dev 2019; 13:133-44. doi: 10.1016/j.omtm.2019.01.001 [Crossref] [ Google Scholar]
- Shtam TA, Kovalev RA, Varfolomeeva EY, Makarov EM, Kil YV, Filatov MV. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun Signal 2013; 11:88. doi: 10.1186/1478-811x-11-88 [Crossref] [ Google Scholar]
- Brossa A, Tapparo M, Fonsato V, Papadimitriou E, Delena M, Camussi G. Coincubation as miR-loading strategy to improve the anti-tumor effect of stem cell-derived EVs. Pharmaceutics 2021; 13(1):76. doi: 10.3390/pharmaceutics13010076 [Crossref] [ Google Scholar]
- Won Lee G, Thangavelu M, Joung Choi M, Yeong Shin E, Sol Kim H, Seon Baek J. Exosome mediated transfer of miRNA-140 promotes enhanced chondrogenic differentiation of bone marrow stem cells for enhanced cartilage repair and regeneration. J Cell Biochem 2020; 121(7):3642-52. doi: 10.1002/jcb.29657 [Crossref] [ Google Scholar]
- Lamichhane TN, Jeyaram A, Patel DB, Parajuli B, Livingston NK, Arumugasaamy N. Oncogene knockdown via active loading of small RNAs into extracellular vesicles by sonication. Cell Mol Bioeng 2016; 9(3):315-24. doi: 10.1007/s12195-016-0457-4 [Crossref] [ Google Scholar]
-
Kempermann G. Adult neurogenesis. In: Pfaff DW, Volkow ND, Rubenstein JL, eds. Neuroscience in the 21st Century: From Basic to Clinical. Cham: Springer International Publishing; 2022. p. 321-39. doi: 10.1007/978-3-030-88832-9_9.
- Migaud M, Batailler M, Segura S, Duittoz A, Franceschini I, Pillon D. Emerging new sites for adult neurogenesis in the mammalian brain: a comparative study between the hypothalamus and the classical neurogenic zones. Eur J Neurosci 2010; 32(12):2042-52. doi: 10.1111/j.1460-9568.2010.07521.x [Crossref] [ Google Scholar]
- Bergmann O, Spalding KL, Frisén J. Adult neurogenesis in humans. Cold Spring Harb Perspect Biol 2015; 7(7):a018994. doi: 10.1101/cshperspect.a018994 [Crossref] [ Google Scholar]
- Kumar A, Pareek V, Faiq MA, Ghosh SK, Kumari C. Adult neurogenesis in humans: a review of basic concepts, history, current research, and clinical implications. Innov Clin Neurosci 2019; 16(5-6):30-7. [ Google Scholar]
- Schuldiner M, Eiges R, Eden A, Yanuka O, Itskovitz-Eldor J, Goldstein RS. Induced neuronal differentiation of human embryonic stem cells. Brain Res 2001; 913(2):201-5. doi: 10.1016/s0006-8993(01)02776-7 [Crossref] [ Google Scholar]
- Dhara SK, Stice SL. Neural differentiation of human embryonic stem cells. J Cell Biochem 2008; 105(3):633-40. doi: 10.1002/jcb.21891 [Crossref] [ Google Scholar]
- Schulz TC, Palmarini GM, Noggle SA, Weiler DA, Mitalipova MM, Condie BG. Directed neuronal differentiation of human embryonic stem cells. BMC Neurosci 2003; 4:27. doi: 10.1186/1471-2202-4-27 [Crossref] [ Google Scholar]
- Carpenter MK, Inokuma MS, Denham J, Mujtaba T, Chiu CP, Rao MS. Enrichment of neurons and neural precursors from human embryonic stem cells. Exp Neurol 2001; 172(2):383-97. doi: 10.1006/exnr.2001.7832 [Crossref] [ Google Scholar]
- Anji A, Anderson B, Akhtar F, Meekins DA, Ito T, Mummidi S. Exosomes induce neurogenesis of pluripotent P19 cells. Stem Cell Rev Rep 2023; 19(5):1152-76. doi: 10.1007/s12015-023-10512-6 [Crossref] [ Google Scholar]
- Luarte A, Cisternas P, Caviedes A, Batiz LF, Lafourcade C, Wyneken U. Astrocytes at the hub of the stress response: potential modulation of neurogenesis by miRNAs in astrocyte-derived exosomes. Stem Cells Int 2017; 2017:1719050. doi: 10.1155/2017/1719050 [Crossref] [ Google Scholar]
- Soni N, Gupta S, Rawat S, Krishnakumar V, Mohanty S, Banerjee A. MicroRNA-enriched exosomes from different sources of mesenchymal stem cells can differentially modulate functions of immune cells and neurogenesis. Biomedicines 2021; 10(1):69. doi: 10.3390/biomedicines10010069 [Crossref] [ Google Scholar]
- Oh HJ. Cell-Non-Autonomous Neurogenesis by Exosome-Mediated Transfer of Neurogenic miRNA in a Microfluidic System [dissertation]. Graduate School of Convergence Science and Technology, Seoul National University; 2016.
- Ma Y, Li C, Huang Y, Wang Y, Xia X, Zheng JC. Exosomes released from neural progenitor cells and induced neural progenitor cells regulate neurogenesis through miR-21a. Cell Commun Signal 2019; 17(1):96. doi: 10.1186/s12964-019-0418-3 [Crossref] [ Google Scholar]
- Yuan P, Ding L, Chen H, Wang Y, Li C, Zhao S. Neural stem cell-derived exosomes regulate neural stem cell differentiation through miR-9-Hes1 axis. Front Cell Dev Biol 2021; 9:601600. doi: 10.3389/fcell.2021.601600 [Crossref] [ Google Scholar]
- Jin T, Gu J, Xia H, Chen H, Xu X, Li Z. Differential expression of microRNA profiles and Wnt signals in stem cell-derived exosomes during dopaminergic neuron differentiation. DNA Cell Biol 2020; 39(12):2143-53. doi: 10.1089/dna.2020.5931 [Crossref] [ Google Scholar]
- Sun X, Chen Y, Zhang Y, Cheng T, Peng H, Sun Y. Exosomes released from immature neurons regulate adult neural stem cell differentiation through microRNA-7a-5p. Stem Cells 2025; 43(2):sxae082. doi: 10.1093/stmcls/sxae082 [Crossref] [ Google Scholar]
- Takeda YS, Xu Q. Neuronal differentiation of human mesenchymal stem cells using exosomes derived from differentiating neuronal cells. PLoS One 2015; 10(8):e0135111. doi: 10.1371/journal.pone.0135111 [Crossref] [ Google Scholar]
- Xu G, Ao R, Zhi Z, Jia J, Yu B. miR-21 and miR-19b delivered by hMSC-derived EVs regulate the apoptosis and differentiation of neurons in patients with spinal cord injury. J Cell Physiol 2019; 234(7):10205-17. doi: 10.1002/jcp.27690 [Crossref] [ Google Scholar]
- Huang JH, Xu Y, Yin XM, Lin FY. Exosomes derived from miR-126-modified MSCs promote angiogenesis and neurogenesis and attenuate apoptosis after spinal cord injury in rats. Neuroscience 2020; 424:133-45. doi: 10.1016/j.neuroscience.2019.10.043 [Crossref] [ Google Scholar]
- Peng D, Liu T, Lu H, Zhang L, Chen H, Huang Y. Intranasal delivery of engineered extracellular vesicles loaded with miR-206-3p antagomir ameliorates Alzheimer’s disease phenotypes. Theranostics 2024; 14(19):7623-44. doi: 10.7150/thno.103596 [Crossref] [ Google Scholar]
- Cafferty WB, McGee AW, Strittmatter SM. Axonal growth therapeutics: regeneration or sprouting or plasticity?. Trends Neurosci 2008; 31(5):215-20. doi: 10.1016/j.tins.2008.02.004 [Crossref] [ Google Scholar]
- Filous AR, Schwab JM. Determinants of axon growth, plasticity, and regeneration in the context of spinal cord injury. Am J Pathol 2018; 188(1):53-62. doi: 10.1016/j.ajpath.2017.09.005 [Crossref] [ Google Scholar]
- Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology 2008; 33(1):18-41. doi: 10.1038/sj.npp.1301559 [Crossref] [ Google Scholar]
- Gogolla N, Galimberti I, Caroni P. Structural plasticity of axon terminals in the adult. Curr Opin Neurobiol 2007; 17(5):516-24. doi: 10.1016/j.conb.2007.09.002 [Crossref] [ Google Scholar]
- Lafourcade C, Ramírez JP, Luarte A, Fernández A, Wyneken U. MiRNAs in astrocyte-derived exosomes as possible mediators of neuronal plasticity. J Exp Neurosci 2016; 10(Suppl 1):1-9. doi: 10.4137/jen.S39916 [Crossref] [ Google Scholar]
- Xin H, Wang F, Li Y, Lu QE, Cheung WL, Zhang Y. Secondary release of exosomes from astrocytes contributes to the increase in neural plasticity and improvement of functional recovery after stroke in rats treated with exosomes harvested from microRNA 133b-overexpressing multipotent mesenchymal stromal cells. Cell Transplant 2017; 26(2):243-57. doi: 10.3727/096368916x693031 [Crossref] [ Google Scholar]
- Wang C, Ji Y, Zhang H, Ye Y, Zhang G, Zhang S. Increased level of exosomal miR-20b-5p derived from hypothermia-treated microglia promotes neurite outgrowth and synapse recovery after traumatic brain injury. Neurobiol Dis 2023; 179:106042. doi: 10.1016/j.nbd.2023.106042 [Crossref] [ Google Scholar]
- Xin H, Katakowski M, Wang F, Qian JY, Liu XS, Ali MM. MicroRNA cluster miR-17-92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke 2017; 48(3):747-53. doi: 10.1161/strokeaha.116.015204 [Crossref] [ Google Scholar]
- Zhang Y, Chopp M, Liu XS, Katakowski M, Wang X, Tian X. Exosomes derived from mesenchymal stromal cells promote axonal growth of cortical neurons. Mol Neurobiol 2017; 54(4):2659-73. doi: 10.1007/s12035-016-9851-0 [Crossref] [ Google Scholar]
- López-Leal R, Díaz-Viraqué F, Catalán RJ, Saquel C, Enright A, Iraola G. Schwann cell reprogramming into repair cells increases miRNA-21 expression in exosomes promoting axonal growth. J Cell Sci 2020; 133(12):jcs239004. doi: 10.1242/jcs.239004 [Crossref] [ Google Scholar]
- Huang W, Lin M, Yang C, Wang F, Zhang M, Gao J. Rat bone mesenchymal stem cell-derived exosomes loaded with miR-494 promoting neurofilament regeneration and behavioral function recovery after spinal cord injury. Oxid Med Cell Longev 2021; 2021:1634917. doi: 10.1155/2021/1634917 [Crossref] [ Google Scholar]
- Ma X, Wang Y, Shi Y, Li S, Liu J, Li X. Exosomal miR-132-3p from mesenchymal stromal cells improves synaptic dysfunction and cognitive decline in vascular dementia. Stem Cell Res Ther 2022; 13(1):315. doi: 10.1186/s13287-022-02995-w [Crossref] [ Google Scholar]
- Gao B, Zhou S, Sun C, Cheng D, Zhang Y, Li X. Brain endothelial cell-derived exosomes induce neuroplasticity in rats with ischemia/reperfusion injury. ACS Chem Neurosci 2020; 11(15):2201-13. doi: 10.1021/acschemneuro.0c00089 [Crossref] [ Google Scholar]
- Xin H, Liu Z, Buller B, Li Y, Golembieski W, Gan X. MiR-17-92 enriched exosomes derived from multipotent mesenchymal stromal cells enhance axon-myelin remodeling and motor electrophysiological recovery after stroke. J Cereb Blood Flow Metab 2021; 41(5):1131-44. doi: 10.1177/0271678x20950489 [Crossref] [ Google Scholar]
- Zhao C, Deng Y, He Y, Huang X, Wang C, Li W. Decreased level of exosomal miR-5121 released from microglia suppresses neurite outgrowth and synapse recovery of neurons following traumatic brain injury. Neurotherapeutics 2021; 18(2):1273-94. doi: 10.1007/s13311-020-00999-z [Crossref] [ Google Scholar]
- Benameur T, Soleti R, Porro C. The potential neuroprotective role of free and encapsulated quercetin mediated by miRNA against neurological diseases. Nutrients 2021; 13(4):1318. doi: 10.3390/nu13041318 [Crossref] [ Google Scholar]
- Zhang D, Cai G, Liu K, Zhuang Z, Jia K, Pei S. Microglia exosomal miRNA-137 attenuates ischemic brain injury through targeting Notch1. Aging (Albany NY) 2021; 13(3):4079-95. doi: 10.18632/aging.202373 [Crossref] [ Google Scholar]
- Aires ID, Ribeiro-Rodrigues T, Boia R, Ferreira-Rodrigues M, Girão H, Ambrósio AF. Microglial extracellular vesicles as vehicles for neurodegeneration spreading. Biomolecules 2021; 11(6):770. doi: 10.3390/biom11060770 [Crossref] [ Google Scholar]
- Hajinejad M, Sahab-Negah S. Neuroinflammation: the next target of exosomal microRNAs derived from mesenchymal stem cells in the context of neurological disorders. J Cell Physiol 2021; 236(12):8070-81. doi: 10.1002/jcp.30495 [Crossref] [ Google Scholar]
- Liang J, Zhu Y, Liu S, Kuang B, Tian Z, Zhang L. Progress of exosomal microRNAs and traditional Chinese medicine monomers in neurodegenerative diseases. Phytother Res 2024; 38(11):5323-49. doi: 10.1002/ptr.8322 [Crossref] [ Google Scholar]
- Burlacu CC, Neag MA, Mitre AO, Sirbu AC, Badulescu AV, Buzoianu AD. The role of miRNAs in dexmedetomidine’s neuroprotective effects against brain disorders. Int J Mol Sci 2022; 23(10):5452. doi: 10.3390/ijms23105452 [Crossref] [ Google Scholar]
- Nasirishargh A, Kumar P, Ramasubramanian L, Clark K, Hao D, Lazar SV. Exosomal microRNAs from mesenchymal stem/stromal cells: biology and applications in neuroprotection. World J Stem Cells 2021; 13(7):776-94. doi: 10.4252/wjsc.v13.i7.776 [Crossref] [ Google Scholar]
- Mavroudis I, Balmus IM, Ciobica A, Nicoara MN, Luca AC, Palade DO. The role of microglial exosomes and miR-124-3p in neuroinflammation and neuronal repair after traumatic brain injury. Life (Basel) 2023; 13(9):1924. doi: 10.3390/life13091924 [Crossref] [ Google Scholar]
- Yang Q, Chen Q, Zhang KB, Liu Y, Zheng JC, Hu DX. Sinomenine alleviates neuroinflammation in chronic cerebral hypoperfusion by promoting M2 microglial polarization and inhibiting neuronal pyroptosis via exosomal miRNA-223-3p. Acta Neuropathol Commun 2025; 13(1):48. doi: 10.1186/s40478-025-01950-z [Crossref] [ Google Scholar]
- Zhou Z, Li C, Bao T, Zhao X, Xiong W, Luo C. Exosome-shuttled miR-672-5p from anti-inflammatory microglia repair traumatic spinal cord injury by inhibiting AIM2/ASC/Caspase-1 signaling pathway mediated neuronal pyroptosis. J Neurotrauma 2022; 39(15-16):1057-74. doi: 10.1089/neu.2021.0464 [Crossref] [ Google Scholar]
- Zhang A, Bai Z, Yi W, Hu Z, Hao J. Overexpression of miR-338-5p in exosomes derived from mesenchymal stromal cells provides neuroprotective effects by the Cnr1/Rap1/Akt pathway after spinal cord injury in rats. Neurosci Lett 2021; 761:136124. doi: 10.1016/j.neulet.2021.136124 [Crossref] [ Google Scholar]
- Yu Z, Wen Y, Jiang N, Li Z, Guan J, Zhang Y. TNF-α stimulation enhances the neuroprotective effects of gingival MSCs derived exosomes in retinal ischemia-reperfusion injury via the MEG3/miR-21a-5p axis. Biomaterials 2022; 284:121484. doi: 10.1016/j.biomaterials.2022.121484 [Crossref] [ Google Scholar]
- Song Y, Li Z, He T, Qu M, Jiang L, Li W. M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics 2019; 9(10):2910-23. doi: 10.7150/thno.30879 [Crossref] [ Google Scholar]
- Ding W, Gu Q, Liu M, Zou J, Sun J, Zhu J. Astrocytes-derived exosomes pre-treated by berberine inhibit neuroinflammation after stroke via miR-182-5p/Rac1 pathway. Int Immunopharmacol 2023; 118:110047. doi: 10.1016/j.intimp.2023.110047 [Crossref] [ Google Scholar]
- Yang Y, Yang H, Yang Y, Ma Y. Exosomal microRNAs have great potential in the neurorestorative therapy for traumatic brain injury. Exp Neurol 2022; 352:114026. doi: 10.1016/j.expneurol.2022.114026 [Crossref] [ Google Scholar]
- Konovalova J, Gerasymchuk D, Parkkinen I, Chmielarz P, Domanskyi A. Interplay between microRNAs and oxidative stress in neurodegenerative diseases. Int J Mol Sci 2019; 20(23):6055. doi: 10.3390/ijms20236055 [Crossref] [ Google Scholar]
- Wang X, Zhou Y, Gao Q, Ping D, Wang Y, Wu W. The role of exosomal microRNAs and oxidative stress in neurodegenerative diseases. Oxid Med Cell Longev 2020; 2020:3232869. doi: 10.1155/2020/3232869 [Crossref] [ Google Scholar]
- Currim F, Shukla S, Singh J, Gohel D, Mane M, Shinde A. Neuronal exosomal miRNAs modulate mitochondrial functions and cell death in bystander neuronal cells under Parkinson’s disease stress conditions. Neurotoxicology 2024; 101:102-16. doi: 10.1016/j.neuro.2024.02.005 [Crossref] [ Google Scholar]
- Abbas A, Huang X, Ullah A, Luo L, Xi W, Qiao Y. Enhanced spinal cord repair using bioengineered induced pluripotent stem cell-derived exosomes loaded with miRNA. Mol Med 2024; 30(1):168. doi: 10.1186/s10020-024-00940-6 [Crossref] [ Google Scholar]
- Wei H, Xu Y, Chen Q, Chen H, Zhu X, Li Y. Mesenchymal stem cell-derived exosomal miR-223 regulates neuronal cell apoptosis. Cell Death Dis 2020; 11(4):290. doi: 10.1038/s41419-020-2490-4 [Crossref] [ Google Scholar]
- Xiao Y, Geng F, Wang G, Li X, Zhu J, Zhu W. Bone marrow-derived mesenchymal stem cells-derived exosomes prevent oligodendrocyte apoptosis through exosomal miR-134 by targeting caspase-8. J Cell Biochem 2019; 120(2):2109-18. doi: 10.1002/jcb.27519 [Crossref] [ Google Scholar]
- Zhang L, Han P. Neural stem cell-derived exosomes suppress neuronal cell apoptosis by activating autophagy via miR-374-5p/STK-4 axis in spinal cord injury. J Musculoskelet Neuronal Interact 2022; 22(3):411-21. [ Google Scholar]
- Cai G, Cai G, Zhou H, Zhuang Z, Liu K, Pei S. Mesenchymal stem cell-derived exosome miR-542-3p suppresses inflammation and prevents cerebral infarction. Stem Cell Res Ther 2021; 12(1):2. doi: 10.1186/s13287-020-02030-w [Crossref] [ Google Scholar]
- Li D, Huang S, Yin Z, Zhu J, Ge X, Han Z. Increases in miR-124-3p in microglial exosomes confer neuroprotective effects by targeting FIP200-mediated neuronal autophagy following traumatic brain injury. Neurochem Res 2019; 44(8):1903-23. doi: 10.1007/s11064-019-02825-1 [Crossref] [ Google Scholar]
- Liu T, Liu H, Xue S, Xiao L, Xu J, Tong S. MiR129-5p-loaded exosomes suppress seizure-associated neurodegeneration in status epilepticus model mice by inhibiting HMGB1/TLR4-mediated neuroinflammation. Mol Biol Rep 2024; 51(1):292. doi: 10.1007/s11033-024-09215-z [Crossref] [ Google Scholar]
- Kumar A, Sidhu J, Lui F, Tsao JW. Alzheimer disease. In: StatPearls [internet]. StatPearls Publishing; 2024.
- Castellani RJ, Rolston RK, Smith MA. Alzheimer disease. Dis Mon 2010; 56(9):484-546. doi: 10.1016/j.disamonth.2010.06.001 [Crossref] [ Google Scholar]
- Lane CA, Hardy J, Schott JM. Alzheimer’s disease. Eur J Neurol 2018; 25(1):59-70. doi: 10.1111/ene.13439 [Crossref] [ Google Scholar]
- Schachter AS, Davis KL. Alzheimer’s disease. Dialogues Clin Neurosci 2000; 2(2):91-100. doi: 10.31887/DCNS.2000.2.2/asschachter [Crossref] [ Google Scholar]
- Long JM, Ray B, Lahiri DK. MicroRNA-153 physiologically inhibits expression of amyloid-β precursor protein in cultured human fetal brain cells and is dysregulated in a subset of Alzheimer disease patients. J Biol Chem 2012; 287(37):31298-310. doi: 10.1074/jbc.M112.366336 [Crossref] [ Google Scholar]
- Long JM, Ray B, Lahiri DK. MicroRNA-339-5p down-regulates protein expression of β-site amyloid precursor protein-cleaving enzyme 1 (BACE1) in human primary brain cultures and is reduced in brain tissue specimens of Alzheimer disease subjects. J Biol Chem 2014; 289(8):5184-98. doi: 10.1074/jbc.M113.518241 [Crossref] [ Google Scholar]
- Gunnels T. Upregulated miRNA-34a Culminates in Decreased TREM2 Expression Influencing the Microglial Response in Alzheimer Disease. 2023. Available from: https://jmsgr.tamhsc.edu/upregulated-mirna-34a-culminates-in-decreased-trem2-expression-influencing-the-microglial-response-in-alzheimer-disease/.
- Chen L, Yang J, Lü J, Cao S, Zhao Q, Yu Z. Identification of aberrant circulating miRNAs in Parkinson’s disease plasma samples. Brain Behav 2018; 8(4):e00941. doi: 10.1002/brb3.941 [Crossref] [ Google Scholar]
- Hill JM, Zhao Y, Clement C, Neumann DM, Lukiw WJ. HSV-1 infection of human brain cells induces miRNA-146a and Alzheimer-type inflammatory signaling. Neuroreport 2009; 20(16):1500-5. doi: 10.1097/WNR.0b013e3283329c05 [Crossref] [ Google Scholar]
- Zhang B, Wang LL, Ren RJ, Dammer EB, Zhang YF, Huang Y. MicroRNA-146a represses LRP2 translation and leads to cell apoptosis in Alzheimer’s disease. FEBS Lett 2016; 590(14):2190-200. doi: 10.1002/1873-3468.12229 [Crossref] [ Google Scholar]
- Galimberti D, Villa C, Fenoglio C, Serpente M, Ghezzi L, Cioffi SM. Circulating miRNAs as potential biomarkers in Alzheimer’s disease. J Alzheimers Dis 2014; 42(4):1261-7. doi: 10.3233/jad-140756 [Crossref] [ Google Scholar]
- Cohen JE, Lee PR, Chen S, Li W, Fields RD. MicroRNA regulation of homeostatic synaptic plasticity. Proc Natl Acad Sci U S A 2011; 108(28):11650-5. doi: 10.1073/pnas.1017576108 [Crossref] [ Google Scholar]
- Schapira AH. Science, medicine, and the future: Parkinson’s disease. BMJ 1999; 318(7179):311-4. doi: 10.1136/bmj.318.7179.311 [Crossref] [ Google Scholar]
- Samii A, Nutt JG, Ransom BR. Parkinson’s disease. Lancet 2004; 363(9423):1783-93. doi: 10.1016/s0140-6736(04)16305-8 [Crossref] [ Google Scholar]
- Kalia LV, Lang AE. Parkinson’s disease. Lancet 2015; 386(9996):896-912. doi: 10.1016/s0140-6736(14)61393-3 [Crossref] [ Google Scholar]
- Lang AE, Lozano AM. Parkinson’s disease Second of two parts. N Engl J Med 1998; 339(16):1130-43. doi: 10.1056/nejm199810153391607 [Crossref] [ Google Scholar]
- Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J. Parkinson disease. Nat Rev Dis Primers 2017; 3:17013. doi: 10.1038/nrdp.2017.13 [Crossref] [ Google Scholar]
- Kim J, Inoue K, Ishii J, Vanti WB, Voronov SV, Murchison E. A microRNA feedback circuit in midbrain dopamine neurons. Science 2007; 317(5842):1220-4. doi: 10.1126/science.1140481 [Crossref] [ Google Scholar]
- Miñones-Moyano E, Porta S, Escaramís G, Rabionet R, Iraola S, Kagerbauer B. MicroRNA profiling of Parkinson’s disease brains identifies early downregulation of miR-34b/c which modulate mitochondrial function. Hum Mol Genet 2011; 20(15):3067-78. doi: 10.1093/hmg/ddr210 [Crossref] [ Google Scholar]
- Liu Y, Song Y, Zhu X. MicroRNA-181a regulates apoptosis and autophagy process in Parkinson’s disease by inhibiting p38 mitogen-activated protein kinase (MAPK)/c-Jun N-terminal kinases (JNK) signaling pathways. Med Sci Monit 2017; 23:1597-606. doi: 10.12659/msm.900218 [Crossref] [ Google Scholar]
- Angelopoulou E, Paudel YN, Piperi C. miR-124 and Parkinson’s disease: a biomarker with therapeutic potential. Pharmacol Res 2019; 150:104515. doi: 10.1016/j.phrs.2019.104515 [Crossref] [ Google Scholar]
- Kabaria S, Choi DC, Chaudhuri AD, Mouradian MM, Junn E. Inhibition of miR-34b and miR-34c enhances α-synuclein expression in Parkinson’s disease. FEBS Lett 2015; 589(3):319-25. doi: 10.1016/j.febslet.2014.12.014 [Crossref] [ Google Scholar]
- Wang H, Ye Y, Zhu Z, Mo L, Lin C, Wang Q. MiR-124 regulates apoptosis and autophagy process in MPTP model of Parkinson’s disease by targeting to Bim. Brain Pathol 2016; 26(2):167-76. doi: 10.1111/bpa.12267 [Crossref] [ Google Scholar]
- Wang G, van der Walt JM, Mayhew G, Li YJ, Züchner S, Scott WK. Variation in the miRNA-433 binding site of FGF20 confers risk for Parkinson disease by overexpression of alpha-synuclein. Am J Hum Genet 2008; 82(2):283-9. doi: 10.1016/j.ajhg.2007.09.021 [Crossref] [ Google Scholar]
- Junn E, Lee KW, Jeong BS, Chan TW, Im JY, Mouradian MM. Repression of alpha-synuclein expression and toxicity by microRNA-7. Proc Natl Acad Sci U S A 2009; 106(31):13052-7. doi: 10.1073/pnas.0906277106 [Crossref] [ Google Scholar]
- Masrori P, Van Damme P. Amyotrophic lateral sclerosis: a clinical review. Eur J Neurol 2020; 27(10):1918-29. doi: 10.1111/ene.14393 [Crossref] [ Google Scholar]
- Phukan J, Pender NP, Hardiman O. Cognitive impairment in amyotrophic lateral sclerosis. Lancet Neurol 2007; 6(11):994-1003. doi: 10.1016/s1474-4422(07)70265-x [Crossref] [ Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998; 51(6):1546-54. doi: 10.1212/wnl.51.6.1546 [Crossref] [ Google Scholar]
- Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O. Amyotrophic lateral sclerosis. Lancet 2011; 377(9769):942-55. doi: 10.1016/s0140-6736(10)61156-7 [Crossref] [ Google Scholar]
- Wijesekera LC, Leigh PN. Amyotrophic lateral sclerosis. Orphanet J Rare Dis 2009; 4:3. doi: 10.1186/1750-1172-4-3 [Crossref] [ Google Scholar]
- Shaw PJ. Molecular and cellular pathways of neurodegeneration in motor neurone disease. J Neurol Neurosurg Psychiatry 2005; 76(8):1046-57. doi: 10.1136/jnnp.2004.048652 [Crossref] [ Google Scholar]
- Averill AJ, Kasarskis EJ, Segerstrom SC. Psychological health in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2007; 8(4):243-54. doi: 10.1080/17482960701374643 [Crossref] [ Google Scholar]
- Hoye ML, Koval ED, Wegener AJ, Hyman TS, Yang C, O’Brien DR. MicroRNA profiling reveals marker of motor neuron disease in ALS models. J Neurosci 2017; 37(22):5574-86. doi: 10.1523/jneurosci.3582-16.2017 [Crossref] [ Google Scholar]
- de Andrade HM, de Albuquerque M, Avansini SH, de Souza Rocha C, Dogini DB, Nucci A. MicroRNAs-424 and 206 are potential prognostic markers in spinal onset amyotrophic lateral sclerosis. J Neurol Sci 2016; 368:19-24. doi: 10.1016/j.jns.2016.06.046 [Crossref] [ Google Scholar]
- De Felice B, Guida M, Guida M, Coppola C, De Mieri G, Cotrufo R. A miRNA signature in leukocytes from sporadic amyotrophic lateral sclerosis. Gene 2012; 508(1):35-40. doi: 10.1016/j.gene.2012.07.058 [Crossref] [ Google Scholar]
- Xu Q, Zhao Y, Zhou X, Luan J, Cui Y, Han J. Comparison of the extraction and determination of serum exosome and miRNA in serum and the detection of miR-27a-3p in serum exosome of ALS patients. Intractable Rare Dis Res 2018; 7(1):13-8. doi: 10.5582/irdr.2017.01091 [Crossref] [ Google Scholar]
- Matamala JM, Arias-Carrasco R, Sanchez C, Uhrig M, Bargsted L, Matus S. Genome-wide circulating microRNA expression profiling reveals potential biomarkers for amyotrophic lateral sclerosis. Neurobiol Aging 2018; 64:123-38. doi: 10.1016/j.neurobiolaging.2017.12.020 [Crossref] [ Google Scholar]
- Vrabec K, Boštjančič E, Koritnik B, Leonardis L, Dolenc Grošelj L, Zidar J. Differential expression of several miRNAs and the host genes AATK and DNM2 in leukocytes of sporadic ALS patients. Front Mol Neurosci 2018; 11:106. doi: 10.3389/fnmol.2018.00106 [Crossref] [ Google Scholar]
- Liguori M, Nuzziello N, Introna A, Consiglio A, Licciulli F, D’Errico E. Dysregulation of microRNAs and target genes networks in peripheral blood of patients with sporadic amyotrophic lateral sclerosis. Front Mol Neurosci 2018; 11:288. doi: 10.3389/fnmol.2018.00288 [Crossref] [ Google Scholar]
- McGinley MP, Goldschmidt CH, Rae-Grant AD. Diagnosis and treatment of multiple sclerosis: a review. JAMA 2021; 325(8):765-79. doi: 10.1001/jama.2020.26858 [Crossref] [ Google Scholar]
- Brust JC. Current Diagnosis and Treatment Neurology. McGraw Hill; 2025.
- Dobson R, Giovannoni G. Multiple sclerosis - a review. Eur J Neurol 2019; 26(1):27-40. doi: 10.1111/ene.13819 [Crossref] [ Google Scholar]
- Goldenberg MM. Multiple sclerosis review. P T 2012; 37(3):175-84. [ Google Scholar]
- Korn T. Pathophysiology of multiple sclerosis. J Neurol 2008; 255 Suppl 6:2-6. doi: 10.1007/s00415-008-6001-2 [Crossref] [ Google Scholar]
- Gajofatto A, Benedetti MD. Treatment strategies for multiple sclerosis: when to start, when to change, when to stop?. World J Clin Cases 2015; 3(7):545-55. doi: 10.12998/wjcc.v3.i7.545 [Crossref] [ Google Scholar]
- Ingwersen J, Menge T, Wingerath B, Kaya D, Graf J, Prozorovski T. Natalizumab restores aberrant miRNA expression profile in multiple sclerosis and reveals a critical role for miR-20b. Ann Clin Transl Neurol 2015; 2(1):43-55. doi: 10.1002/acn3.152 [Crossref] [ Google Scholar]
- Tripathi A, Volsko C, Datta U, Regev K, Dutta R. Expression of disease-related miRNAs in white-matter lesions of progressive multiple sclerosis brains. Ann Clin Transl Neurol 2019; 6(5):854-62. doi: 10.1002/acn3.750 [Crossref] [ Google Scholar]
- Vistbakka J, Sumelahti ML, Lehtimäki T, Elovaara I, Hagman S. Evaluation of serum miR-191-5p, miR-24-3p, miR-128-3p, and miR-376c-3 in multiple sclerosis patients. Acta Neurol Scand 2018; 138(2):130-6. doi: 10.1111/ane.12921 [Crossref] [ Google Scholar]
- Keller A, Leidinger P, Steinmeyer F, Stähler C, Franke A, Hemmrich-Stanisak G. Comprehensive analysis of microRNA profiles in multiple sclerosis including next-generation sequencing. Mult Scler 2014; 20(3):295-303. doi: 10.1177/1352458513496343 [Crossref] [ Google Scholar]
- Ali Ashrafi S, Asadi M, Shanehbandi D, Sadigh Eteghad S, Fazlollahi A, Nejadghaderi SA. Association between miRNA-145 and miRNA-155 expression in peripheral blood mononuclear cells of patients with multiple sclerosis: a case-control study. BMC Neurol 2022; 22(1):405. doi: 10.1186/s12883-022-02909-6 [Crossref] [ Google Scholar]
- Søndergaard HB, Hesse D, Krakauer M, Sørensen PS, Sellebjerg F. Differential microRNA expression in blood in multiple sclerosis. Mult Scler 2013; 19(14):1849-57. doi: 10.1177/1352458513490542 [Crossref] [ Google Scholar]
- Rhead B, Shao X, Graves JS, Chitnis T, Waldman AT, Lotze T. miRNA contributions to pediatric-onset multiple sclerosis inferred from GWAS. Ann Clin Transl Neurol 2019; 6(6):1053-61. doi: 10.1002/acn3.786 [Crossref] [ Google Scholar]
- Quadri SA, Farooqui M, Ikram A, Zafar A, Khan MA, Suriya SS. Recent update on basic mechanisms of spinal cord injury. Neurosurg Rev 2020; 43(2):425-41. doi: 10.1007/s10143-018-1008-3 [Crossref] [ Google Scholar]
- Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT, Ellegala DB. Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol 2001; 24(5):254-64. doi: 10.1097/00002826-200109000-00002 [Crossref] [ Google Scholar]
- Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A. Traumatic spinal cord injury. Nat Rev Dis Primers 2017; 3:17018. doi: 10.1038/nrdp.2017.18 [Crossref] [ Google Scholar]
- Chen Y, Tang Y, Vogel LC, Devivo MJ. Causes of spinal cord injury. Top Spinal Cord Inj Rehabil 2013; 19(1):1-8. doi: 10.1310/sci1901-1 [Crossref] [ Google Scholar]
- Simpson LA, Eng JJ, Hsieh JT, Wolfe DL. The health and life priorities of individuals with spinal cord injury: a systematic review. J Neurotrauma 2012; 29(8):1548-55. doi: 10.1089/neu.2011.2226 [Crossref] [ Google Scholar]
- Venkatesh K, Ghosh SK, Mullick M, Manivasagam G, Sen D. Spinal cord injury: pathophysiology, treatment strategies, associated challenges, and future implications. Cell Tissue Res 2019; 377(2):125-51. doi: 10.1007/s00441-019-03039-1 [Crossref] [ Google Scholar]
- Hu JZ, Huang JH, Zeng L, Wang G, Cao M, Lu HB. Anti-apoptotic effect of microRNA-21 after contusion spinal cord injury in rats. J Neurotrauma 2013; 30(15):1349-60. doi: 10.1089/neu.2012.2748 [Crossref] [ Google Scholar]
- Shi Z, Zhou H, Lu L, Li X, Fu Z, Liu J. The roles of microRNAs in spinal cord injury. Int J Neurosci 2017; 127(12):1104-15. doi: 10.1080/00207454.2017.1323208 [Crossref] [ Google Scholar]
- Yu T, Zhao C, Hou S, Zhou W, Wang B, Chen Y. Exosomes secreted from miRNA-29b-modified mesenchymal stem cells repaired spinal cord injury in rats. Braz J Med Biol Res 2019; 52(12):e8735. doi: 10.1590/1414-431x20198735 [Crossref] [ Google Scholar]
- Pinchi E, Frati A, Cantatore S, D’Errico S, Russa R, Maiese A. Acute spinal cord injury: a systematic review investigating miRNA families involved. Int J Mol Sci 2019; 20(8):1841. doi: 10.3390/ijms20081841 [Crossref] [ Google Scholar]
- Nakanishi K, Nakasa T, Tanaka N, Ishikawa M, Yamada K, Yamasaki K. Responses of microRNAs 124a and 223 following spinal cord injury in mice. Spinal Cord 2010; 48(3):192-6. doi: 10.1038/sc.2009.89 [Crossref] [ Google Scholar]
- Jee MK, Jung JS, Choi JI, Jang JA, Kang KS, Im YB. MicroRNA 486 is a potentially novel target for the treatment of spinal cord injury. Brain 2012; 135(Pt 4):1237-52. doi: 10.1093/brain/aws047 [Crossref] [ Google Scholar]
- Liu NK, Wang XF, Lu QB, Xu XM. Altered microRNA expression following traumatic spinal cord injury. Exp Neurol 2009; 219(2):424-9. doi: 10.1016/j.expneurol.2009.06.015 [Crossref] [ Google Scholar]
- Walker FO. Huntington’s disease. Lancet 2007; 369(9557):218-28. doi: 10.1016/s0140-6736(07)60111-1 [Crossref] [ Google Scholar]
- Bates G, Tabrizi S, Jones L. Huntington’s Disease. Oxford University Press; 2014.
- Ghosh R, Tabrizi S. Huntington disease. Handb Clin Neurol 2018; 147:255-78. doi: 10.1016/b978-0-444-63233-3.00017-8 [Crossref] [ Google Scholar]
- Shiwal A, Nibrad D, Tadas M, Katariya R, Kale M, Wankhede N. Polyamines signalling pathway: a key player in unveiling the molecular mechanisms underlying Huntington’s disease. Neuroscience 2025; 570:213-24. doi: 10.1016/j.neuroscience.2025.02.042 [Crossref] [ Google Scholar]
- Ajitkumar A, De Jesus O. Huntington disease. In: StatPearls [Internet]. StatPearls Publishing; 2023.
- Caron NS, Wright GE, Hayden MR. Huntington disease. In: GeneReviews®[Internet]. Seattle, WA: University of Washington, Seattle; 2020.
- Caron NS, Southwell AL, Brouwers CC, Cengio LD, Xie Y, Black HF. Potent and sustained huntingtin lowering via AAV5 encoding miRNA preserves striatal volume and cognitive function in a humanized mouse model of Huntington disease. Nucleic Acids Res 2020; 48(1):36-54. doi: 10.1093/nar/gkz976 [Crossref] [ Google Scholar]
- Reed ER, Latourelle JC, Bockholt JH, Bregu J, Smock J, Paulsen JS. MicroRNAs in CSF as prodromal biomarkers for Huntington disease in the PREDICT-HD study. Neurology 2018; 90(4):e264-72. doi: 10.1212/wnl.0000000000004844 [Crossref] [ Google Scholar]
- Oh YM, Lee SW, Yoo AS. Modeling Huntington disease through microRNA-mediated neuronal reprogramming identifies age-associated autophagy dysfunction driving the onset of neurodegeneration. Autophagy 2023; 19(9):2613-5. doi: 10.1080/15548627.2023.2175572 [Crossref] [ Google Scholar]
- Kozlowska E, Krzyzosiak WJ, Koscianska E. Regulation of huntingtin gene expression by miRNA-137, -214, -148a, and their respective isomiRs. Int J Mol Sci 2013; 14(8):16999-7016. doi: 10.3390/ijms140816999 [Crossref] [ Google Scholar]
- Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 2015; 4:27066. doi: 10.3402/jev.v4.27066 [Crossref] [ Google Scholar]
- Syromiatnikova V, Prokopeva A, Gomzikova M. Methods of the large-scale production of extracellular vesicles. Int J Mol Sci 2022; 23(18):10522. doi: 10.3390/ijms231810522 [Crossref] [ Google Scholar]
- Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomedicine 2020; 15:6917-34. doi: 10.2147/ijn.S264498 [Crossref] [ Google Scholar]
- Palakurthi SS, Shah B, Kapre S, Charbe N, Immanuel S, Pasham S. A comprehensive review of challenges and advances in exosome-based drug delivery systems. Nanoscale Adv 2024; 6(23):5803-26. doi: 10.1039/d4na00501e [Crossref] [ Google Scholar]
- Wang J, Chen D, Ho EA. Challenges in the development and establishment of exosome-based drug delivery systems. J Control Release 2021; 329:894-906. doi: 10.1016/j.jconrel.2020.10.020 [Crossref] [ Google Scholar]
- Lee M, Ban JJ, Im W, Kim M. Influence of storage condition on exosome recovery. Biotechnol Bioprocess Eng 2016; 21(2):299-304. doi: 10.1007/s12257-015-0781-x [Crossref] [ Google Scholar]
- Agrawal AK, Aqil F, Jeyabalan J, Spencer WA, Beck J, Gachuki BW. Milk-derived exosomes for oral delivery of paclitaxel. Nanomedicine 2017; 13(5):1627-36. doi: 10.1016/j.nano.2017.03.001 [Crossref] [ Google Scholar]
- Sokolova V, Ludwig AK, Hornung S, Rotan O, Horn PA, Epple M. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf B Biointerfaces 2011; 87(1):146-50. doi: 10.1016/j.colsurfb.2011.05.013 [Crossref] [ Google Scholar]
- Ruzycka-Ayoush M, Nowicka AM, Kowalczyk A, Gluchowska A, Targonska A, Mosieniak G. Exosomes derived from lung cancer cells: isolation, characterization, and stability studies. Eur J Pharm Sci 2023; 181:106369. doi: 10.1016/j.ejps.2022.106369 [Crossref] [ Google Scholar]
- Lőrincz Á M, Timár CI, Marosvári KA, Veres DS, Otrokocsi L, Kittel Á. Effect of storage on physical and functional properties of extracellular vesicles derived from neutrophilic granulocytes. J Extracell Vesicles 2014; 3:25465. doi: 10.3402/jev.v3.25465 [Crossref] [ Google Scholar]
- Wang B, Zhuang X, Deng ZB, Jiang H, Mu J, Wang Q. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol Ther 2014; 22(3):522-34. doi: 10.1038/mt.2013.190 [Crossref] [ Google Scholar]
- Lener T, Gimona M, Aigner L, Börger V, Buzas E, Camussi G. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J Extracell Vesicles 2015; 4:30087. doi: 10.3402/jev.v4.30087 [Crossref] [ Google Scholar]
- Buschmann D, Kirchner B, Hermann S, Märte M, Wurmser C, Brandes F. Evaluation of serum extracellular vesicle isolation methods for profiling miRNAs by next-generation sequencing. J Extracell Vesicles 2018; 7(1):1481321. doi: 10.1080/20013078.2018.1481321 [Crossref] [ Google Scholar]
- Tang Y, Zhou Y, Li HJ. Advances in mesenchymal stem cell exosomes: a review. Stem Cell Res Ther 2021; 12(1):71. doi: 10.1186/s13287-021-02138-7 [Crossref] [ Google Scholar]
- Chen J, Li P, Zhang T, Xu Z, Huang X, Wang R. Review on strategies and technologies for exosome isolation and purification. Front Bioeng Biotechnol 2021; 9:811971. doi: 10.3389/fbioe.2021.811971 [Crossref] [ Google Scholar]
- Zhang M, Jin K, Gao L, Zhang Z, Li F, Zhou F. Methods and technologies for exosome isolation and characterization. Small Methods 2018; 2(9):1800021. doi: 10.1002/smtd.201800021 [Crossref] [ Google Scholar]
- Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 2019; 8(7):727. doi: 10.3390/cells8070727 [Crossref] [ Google Scholar]
-
Zhou M, Weber SR, Zhao Y, Chen H, Sundstrom JM. Methods for exosome isolation and characterization. In: Edelstein L, Smythies J, Quesenberry P, Noble D, eds. Exosomes. Academic Press; 2020. p. 23-38. doi: 10.1016/b978-0-12-816053-4.00002-x.
- Amiri A, Bagherifar R, Ansari Dezfouli E, Kiaie SH, Jafari R, Ramezani R. Exosomes as bio-inspired nanocarriers for RNA delivery: preparation and applications. J Transl Med 2022; 20(1):125. doi: 10.1186/s12967-022-03325-7 [Crossref] [ Google Scholar]
- Lai JJ, Chau ZL, Chen SY, Hill JJ, Korpany KV, Liang NW. Exosome processing and characterization approaches for research and technology development. Adv Sci (Weinh) 2022; 9(15):e2103222. doi: 10.1002/advs.202103222 [Crossref] [ Google Scholar]
- Ayala-Mar S, Donoso-Quezada J, Gallo-Villanueva RC, Perez-Gonzalez VH, González-Valdez J. Recent advances and challenges in the recovery and purification of cellular exosomes. Electrophoresis 2019; 40(23-24):3036-49. doi: 10.1002/elps.201800526 [Crossref] [ Google Scholar]
- Choi H, Choi K, Kim DH, Oh BK, Yim H, Jo S. Strategies for targeted delivery of exosomes to the brain: advantages and challenges. Pharmaceutics 2022; 14(3):672. doi: 10.3390/pharmaceutics14030672 [Crossref] [ Google Scholar]
- Heidarzadeh M, Gürsoy-Özdemir Y, Kaya M, Eslami Abriz A, Zarebkohan A, Rahbarghazi R. Exosomal delivery of therapeutic modulators through the blood-brain barrier; promise and pitfalls. Cell Biosci 2021; 11(1):142. doi: 10.1186/s13578-021-00650-0 [Crossref] [ Google Scholar]
-
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science 2020;367(6478). doi: 10.1126/science.aau6977.
- Bashyal S, Thapa C, Lee S. Recent progresses in exosome-based systems for targeted drug delivery to the brain. J Control Release 2022; 348:723-44. doi: 10.1016/j.jconrel.2022.06.011 [Crossref] [ Google Scholar]
- Elliott RO, He M. Unlocking the power of exosomes for crossing biological barriers in drug delivery. Pharmaceutics 2021; 13(1):122. doi: 10.3390/pharmaceutics13010122 [Crossref] [ Google Scholar]
- Khatami SH, Karami N, Taheri-Anganeh M, Taghvimi S, Tondro G, Khorsand M. Exosomes: promising delivery tools for overcoming blood-brain barrier and glioblastoma therapy. Mol Neurobiol 2023; 60(8):4659-78. doi: 10.1007/s12035-023-03365-0 [Crossref] [ Google Scholar]
- Rezaie J, Feghhi M, Etemadi T. A review on exosomes application in clinical trials: perspective, questions, and challenges. Cell Commun Signal 2022; 20(1):145. doi: 10.1186/s12964-022-00959-4 [Crossref] [ Google Scholar]
- Clinicaltrials website. 2025. Available from: https://clinicaltrials.gov/.
- Aslan C, Kiaie SH, Majidi Zolbanin N, Lotfinejad P, Ramezani R, Kashanchi F. Exosomes for mRNA delivery: a novel biotherapeutic strategy with hurdles and hope. BMC Biotechnol 2021; 21(1):20. doi: 10.1186/s12896-021-00683-w [Crossref] [ Google Scholar]
- Lu Y, Huang W, Li M, Zheng A. Exosome-based carrier for RNA delivery: progress and challenges. Pharmaceutics 2023; 15(2):598. doi: 10.3390/pharmaceutics15020598 [Crossref] [ Google Scholar]
- Tsai SJ, Atai NA, Cacciottolo M, Nice J, Salehi A, Guo C. Exosome-mediated mRNA delivery in vivo is safe and can be used to induce SARS-CoV-2 immunity. J Biol Chem 2021; 297(5):101266. doi: 10.1016/j.jbc.2021.101266 [Crossref] [ Google Scholar]
- Tzng E, Bayardo N, Yang PC. Current challenges surrounding exosome treatments. Extracell Vesicle 2023; 2:100023. doi: 10.1016/j.vesic.2023.100023 [Crossref] [ Google Scholar]
- Jain KK. Personalized neurology. Per Med 2005; 2(1):15-21. doi: 10.1517/17410541.2.1.15 [Crossref] [ Google Scholar]
- Zhong L, Wang J, Wang P, Liu X, Liu P, Cheng X. Neural stem cell-derived exosomes and regeneration: cell-free therapeutic strategies for traumatic brain injury. Stem Cell Res Ther 2023; 14(1):198. doi: 10.1186/s13287-023-03409-1 [Crossref] [ Google Scholar]
-
Jain KK. Personalized therapy of neurological disorders. In: Textbook of Personalized Medicine. Cham: Springer International Publishing; 2021. p. 213-62. doi: 10.1007/978-3-030-62080-6_11.
- Minocha N, Sardana S. Exosome and other extracellular vesicles in gene therapy and personalized care. Curr Pharmacogenomics Person Med 2023; 20(3):135-45. doi: 10.2174/0118756921285480240118051820 [Crossref] [ Google Scholar]
- Bougea A, Gourzis P. Biomarker-based precision therapy for Alzheimer’s disease: multidimensional evidence leading a new breakthrough in personalized medicine. J Clin Med 2024; 13(16):4661. doi: 10.3390/jcm13164661 [Crossref] [ Google Scholar]
- Park J, Choi Y. Exosome identification for personalized diagnosis and therapy. Biome Eng Lett 2014; 4(3):258-68. doi: 10.1007/s13534-014-0152-0 [Crossref] [ Google Scholar]
- Mehdizadeh S, Mamaghani M, Hassanikia S, Pilehvar Y, Ertas YN. Exosome-powered neuropharmaceutics: unlocking the blood-brain barrier for next-gen therapies. J Nanobiotechnology 2025; 23(1):329. doi: 10.1186/s12951-025-03352-8 [Crossref] [ Google Scholar]
- Guo L, Pan J, Li F, Zhao L, Shi Y. A novel brain targeted plasma exosomes enhance the neuroprotective efficacy of edaravone in ischemic stroke. IET Nanobiotechnol 2021; 15(1):107-16. doi: 10.1049/nbt2.12003 [Crossref] [ Google Scholar]
- Liu X, Hao Y, Huang Z, Shi Y, Su C, Zhao L. Modulation of microglial polarization by sequential targeting surface-engineered exosomes improves therapy for ischemic stroke. Drug Deliv Transl Res 2024; 14(2):418-32. doi: 10.1007/s13346-023-01408-6 [Crossref] [ Google Scholar]
- Kalamakis G, Platt RJ. CRISPR for neuroscientists. Neuron 2023; 111(15):2282-311. doi: 10.1016/j.neuron.2023.04.021 [Crossref] [ Google Scholar]
- Kaminski MM, Abudayyeh OO, Gootenberg JS, Zhang F, Collins JJ. CRISPR-based diagnostics. Nat Biomed Eng 2021; 5(7):643-56. doi: 10.1038/s41551-021-00760-7 [Crossref] [ Google Scholar]
- Garcia-Guerra A, Sathyaprakash C, de Jong OG, Lim WF, Vader P, El Andaloussi S. Tissue-specific modulation of CRISPR activity by miRNA-sensing guide RNAs. Nucleic Acids Res 2025; 53(2):gkaf016. doi: 10.1093/nar/gkaf016 [Crossref] [ Google Scholar]
- Yaghoobi A, Nazerian Y, Zeinaddini Meymand A, Ansari A, Nazerian A, Niknejad H. Hypoxia-sensitive miRNA regulation via CRISPR/dCas9 loaded in hybrid exosomes: a novel strategy to improve embryo implantation and prevent placental insufficiency during pregnancy. Front Cell Dev Biol 2022; 10:1082657. doi: 10.3389/fcell.2022.1082657 [Crossref] [ Google Scholar]
- Golchin A, Shams F, Basiri A, Ranjbarvan P, Kiani S, Sarkhosh-Inanlou R. Combination therapy of stem cell-derived exosomes and biomaterials in the wound healing. Stem Cell Rev Rep 2022; 18(6):1892-911. doi: 10.1007/s12015-021-10309-5 [Crossref] [ Google Scholar]
- Zhang X, Jiang W, Lu Y, Mao T, Gu Y, Ju D. Exosomes combined with biomaterials in the treatment of spinal cord injury. Front Bioeng Biotechnol 2023; 11:1077825. doi: 10.3389/fbioe.2023.1077825 [Crossref] [ Google Scholar]
- Ojeda-Hernández DD, Hernández-Sapiéns MA, Reza-Zaldívar EE, Canales-Aguirre A, Matías-Guiu JA, Matías-Guiu J. Exosomes and biomaterials: in search of a new therapeutic strategy for multiple sclerosis. Life (Basel) 2022; 12(9):1417. doi: 10.3390/life12091417 [Crossref] [ Google Scholar]
- Yadav K, Vijayalakshmi R, Kumar Sahu K, Sure P, Chahal K, Yadav R. Exosome-based macromolecular neurotherapeutic drug delivery approaches in overcoming the blood-brain barrier for treating brain disorders. Eur J Pharm Biopharm 2024; 199:114298. doi: 10.1016/j.ejpb.2024.114298 [Crossref] [ Google Scholar]
- Zhai Y, He F, Fang J, Li S. Advances in the combination of stem cell exosomes with medical devices-the new direction for combination products. Chin J Nat Med 2024; 22(12):1067-75. doi: 10.1016/s1875-5364(24)60637-0 [Crossref] [ Google Scholar]
- Gangadaran P, Hong CM, Ahn BC. An update on in vivo imaging of extracellular vesicles as drug delivery vehicles. Front Pharmacol 2018; 9:169. doi: 10.3389/fphar.2018.00169 [Crossref] [ Google Scholar]
- Petroni D, Fabbri C, Babboni S, Menichetti L, Basta G, Del Turco S. Extracellular vesicles and intercellular communication: challenges for in vivo molecular imaging and tracking. Pharmaceutics 2023; 15(6):1639. doi: 10.3390/pharmaceutics15061639 [Crossref] [ Google Scholar]
- Kim JE, Kalimuthu S, Ahn BC. In vivo cell tracking with bioluminescence imaging. Nucl Med Mol Imaging 2015; 49(1):3-10. doi: 10.1007/s13139-014-0309-x [Crossref] [ Google Scholar]
-
Verweij FJ, Revenu C, Arras G, Dingli F, Loew D, Pegtel DM, et al. Live tracking of inter-organ communication by endogenous exosomes in vivo. Dev Cell 2019;48(4):573-89.e4. doi: 10.1016/j.devcel.2019.01.004.
- Zomer A, Maynard C, Verweij FJ, Kamermans A, Schäfer R, Beerling E. In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell 2015; 161(5):1046-57. doi: 10.1016/j.cell.2015.04.042 [Crossref] [ Google Scholar]
- Lai CP, Kim EY, Badr CE, Weissleder R, Mempel TR, Tannous BA. Visualization and tracking of tumour extracellular vesicle delivery and RNA translation using multiplexed reporters. Nat Commun 2015; 6:7029. doi: 10.1038/ncomms8029 [Crossref] [ Google Scholar]
- Hu L, Wickline SA, Hood JL. Magnetic resonance imaging of melanoma exosomes in lymph nodes. Magn Reson Med 2015; 74(1):266-71. doi: 10.1002/mrm.25376 [Crossref] [ Google Scholar]
- Busato A, Bonafede R, Bontempi P, Scambi I, Schiaffino L, Benati D. Magnetic resonance imaging of ultrasmall superparamagnetic iron oxide-labeled exosomes from stem cells: a new method to obtain labeled exosomes. Int J Nanomedicine 2016; 11:2481-90. doi: 10.2147/ijn.S104152 [Crossref] [ Google Scholar]