Biomed adv. 2(3):131-136.
doi: 10.34172/bma.26
Review Article
New developments in epigenetic and genetic biomarkers for differentiated thyroid cancer (DTC): Moving toward accurate diagnosis and treatment by 2025
Samaneh Hosseinzadeh Conceptualization, Investigation, Supervision, Validation, Visualization, 
Safura Pakizehkar Methodology, Visualization, Writing – original draft,
Hoda Golab-Ghadaksaz Methodology, Visualization, Writing – original draft,
Laleh Hoghooghi Rad Methodology, Visualization, Writing – original draft,
Mehdi Hedayati Conceptualization, Investigation, Supervision, Validation, Visualization, Writing – review & editing, * 
Author information:
Cellular and Molecular Endocrine Research Center, Research Institute for Endocrine Molecular Biology, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Abstract
Summary
Differentiated thyroid carcinoma (DTC), such as papillary and follicular carcinoma of the thyroid, has rising global incidence. While most present with extremely favorable prognosis, some poor prognostic subgroups develop recurrence and become resistant to radioactive iodine (RAI), urgently requiring improved prognostic markers and diagnostic modalities. Recent advances in molecular biology have identified critical biomarkers such as BRAF V600E mutations (40–60% PTC), TERT promoter mutations (10–20% DTC), and epigenetic alterations—that refine risk stratification and guide treatment decisions. The biomarkers make personalized approaches feasible, maximizing management of patients with minimal overtreatment. New tools such as liquid biopsy and next-generation sequencing (NGS) are further augmenting precision medicine strategies. This article cites recent advancements in molecular biomarkers and their revolutionary role in addressing the heterogeneity of DTC.
Keywords: Differentiated thyroid cancer (DTC), Genetic mutations, Epigenetic alterations, Non-coding RNAs, Liquid biopsy, Radioactive iodine resistance, Targeted therapy, Precision medicine
Copyright and License Information
© 2025 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
None.
Introduction
Differentiated thyroid carcinoma (DTC) is the most common form of thyroid cancer, making up more than 90% of cases and is defined by its development from follicular epithelial cells. Most cases of DTC, which include papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma (FTC), are slow growing and have a survivability of greater than 98% within a 5-year span. Unfortunately, 5–10% of patients do progress to metastatic disease, and nearly half of those become resistant to treatment via radioactive iodine (RAI), resulting in an overall dire prognosis with a 10-year survival rate of approximately 10 %.1 In contrast to more modern approaches, traditional risk estimation is based purely on clinicopathological aspects, like tumor size, lymph node metastasis, and even distant metastasis.2 Unfortunately, many of these parameters do not respond to the molecular complexity that drives the behavior of DTCs, leading to the issue of inappropriate treatment.2
Thanks to the development of next generation sequencing (NGS), the capabilities of liquid biopsy procedures, and high-levels of precision in immunohistochemistry, there has been a shift in understanding the molecular aspects underpinning DTC.3 Significantly relevant pathways, such as the mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K)-AKT pathways, are modified with far greater frequency than these of epigenetic changes or non-coding RNA alterations, and alongside such, dysregulation of non-coding RNA also takes place.4,5 This form of review focuses on the molecular biomarkers for the relevant type of DTC, placing the accent on improving the marker definition, outlining progression expectations, and personalizing strategies from treatment up to March 30, 2025.2
Genetic mutations as biomarkers
BRAF V600E mutation
The hallmark feature of PTC stratified by BRAF V600E mutation impacting an astounding 40% to 60% of patients partitions the spindle BRAF Kinase in conjunction with constitutive activation leading to mitogen activated protein kinase (MAPK) signaling cascade. This results in the phosphorylation of MEK and ERK, which enhances the processes of cell proliferation, survival, and dedifferentiation.6-8 It is worth noticing that occurrence of this mutation is marked in Classic variant of PTC which is also known for possessing extra-thyroidal extension, Lymph Node Metastasis, RAI refractoriness.5,8,9
It is important to mention that BRAF V600E exacerbates redevelopment in more advanced subtypes referred to as tall-cell variant exacerbating its role as a prognostic value.6,8,9 A step forward in liquid biopsy technology has advanced the identification of BRAF V600E mutation, leading to a non-invasive way of monitoring the cancer and the patient’s response to treatment.7,10
Currently, BRAF inhibitors such as dabrafenib, in conjunction with MEK inhibitors like trametinib, have been proven effective in treating BRAF-mutated RAI-refractory DTCs, with the best objective response rates standing at 60%.10-12 However, the current treatment has its limitation, involving the development of resistance after continuous drug exposure, such as MAPK pathway resistance, or cross-resistance mainly due to PI3K-AKT pathway activation.12-14 Scientists have therefore devised research plans to find long-term solutions to the drug resistance by developing newer generations of BRAF inhibitors to prevent widespread activation of the former or to combine BRAF and MEK inhibitors with immune checkpoint blockade to limit tumor.10,12,14
RAS mutations
RAS genes mutations (NRAS, HRAS, and KRAS) are detected in 10–20% of PTC and 30–40% of FTC, and are related with both MAPK and PI3K-AKT pathways.16 RAS mutations cause the presence of a constitutively active form of RAS proteins, which typically function as molecular switches by turning on and off numerous signal transduction pathways such as RAF, PI3K, and AKT. This results in enhanced cell proliferation, survival, and reprogramming of cell metabolism.15
Tumors with RAS mutations usually feature follicular patterns of growth and are connected with encapsulated or minimally invasive FTC. Such tumors are usually less harmful compared to those driven by BRAF, with a lower incidence of lymphatic node metastasis and extra-thyroidal expansion. However, mutations in RAS have been associated with a greater likelihood of tumor dedifferentiation, which results in anaplastic or poorly DTC in some instances.15
Updated information implies that mutations in RAS genes are indicators of the intermediate prognosis category and assist in determining the intensity of RAI therapy. In contrast to BRAF V600E mutations, RAS mutations generally lead to a more stable clinical course, highlighting the need for a different therapeutic approach. RAS-mutated tumors are less aggressive than BRAF V600E-driven cases, so the former is less likely to exhibit rapid progression. Even though tumors with RAS mutations typically have a better prognosis, they need to be closely monitored to detect any signs of dedifferentiation and progression.15
At the moment, there are no approved therapies by the FDA designed to address RAS mutations. Nonetheless, upcoming treatment options focus on MEK, PI3K, and mTOR targeting downstream effectors as essential solutions for RAS-mutated DTC.15 These combination treatments might provide new solutions to address these tumors, especially where conventional options have become ineffective.15
TERT promoter mutations
Telomerase reverse transcriptase (TERT) promoter mutations (C228T and C250T) are identified in 10–20% of DTC cases and are firmly associated with aggressive conduct, distal metastasis, and lessened endurance. These mutations bring about new binding sites for proteins such as ETS/TCF, which boost TERT expression. A rise in telomerase activity helps cancer cells preserve telomere length, encouraging cellular longevity and tumorigenesis. TERT promoter changes are linked with a poorer prognosis, such as decreased survival without tumor recurrence and overall survival. Furthermore, if these alterations co-occur with BRAF V600E or RAS mutations, the likelihood of an aggressive disease and dying from it rises. For instance, when patients have both BRAF V600E and TERT promoter mutations, their chances of full recovery and survival are much lower than if they only have one of these mutations. Integrating TERT testing into the newer NGS panels helps in early detection of high-risk patients. This stands critical to modify the treatment approach either through an increase in RAI therapy or considering targeted therapies in refractory cases.9
Direct targeting of TERT has its challenges due to its significant role in regular cellular functions. Nonetheless, new strategies lie in the inhibition of downstream pathways upregulated by TERT, for instance, MAPK and PI3K-AKT signaling. Moreover, therapeutic approaches using immune system targeting telomerase-specific antigens are being discovered as a way of individualized medical care in this high-risk group. Constant studies are presently researching combined methods that direct both TERT-related processes and immune checkpoint suppression. This strategy targets resistance and the survival of TERT-mutated malignancies patients and boosts the results. At the time when the knowledge of TERT genetics is improving, TERT-applied gene is considered to be a significant element of risk categorization and medical treatment of TERT-mutated malignancies.9
Gene fusions (RET, NTRK, ALK)
A fusion of genes in RET, NTRK and ALK occurs in 5–15% of DTCs, mainly in young patients or post-radiation disease. It is produced by the rearrangement of chromosomes generating oncogenetic fusion proteins that guarantee the constitutive activation of many signaling pathways, including the MAPK signaling system, the PI3K-AKT signaling system, and tyrosine protein kinase pathways. RET fusions are frequently seen in radiation-induced PTC, while NTRK fusions are noted in a variety of histologic subtypes, including PTC and poorly differentiated thyroid carcinoma (PDTC). ALK fusions, although infrequent, occur more often in cases that are aggressive or nonresponsive.16,17
The adjustments are actionable because there are FDA-approved inhibitors available for treatment. An instance in point, Retevmo has displayed extraordinary potency in RET amalgamation-positive thyroidal cancer, obtaining an objective response rate in RET-mutant thyroidal cancer (MTC) and in RET fusion-positive DTC in the LIBRETTO-001 trial.17 Also, Vitrakvi, a pan-NTRK inhibitor, obtained an ORR of 75% in NTRK fusion-positive cancers, underlining its potential in thyroidal cancer management. Other than RET and NTRK fusions, ALK fusions have been identified as another viable target. Crizotinib and alectinib, ALK inhibitors, have shown assurance in the management of ALK-positive cancers, underlining the value of overall DNA sequencing in detecting unique but highly clinically relevant mutations.18
Even though they are effective, acquired resistance mechanisms such as other mutations in the same kinase domain or bypass signaling pathways emphasize the necessity for therapies in combination. More recent inhibitors and combining agents are being studied in ongoing research to overcome resistance mechanisms. In another approach, therapeutic options of immunotherapy against fusion-specific neoantigens are being investigated, opening up a possibility of precision therapy in fusion-positive tumors.18 The use of comprehensive genomic profiling through RNA sequencing is one of the mainstays of precision medicine in DTC helping in early detection of fusion-positive cases; this data is essential for designing therapeutic strategies especially in refractory cases or those which have turned metastatic where targeted therapies can make monumental differences. With the evolution of our knowledge about gene fusions, we think that it will start to play a more and more significant role in determining risk categories and decisions related to treatment.18
Figure 1 illustrates the approximate frequency and clinical characteristics of major genetic biomarkers associated with DTC.
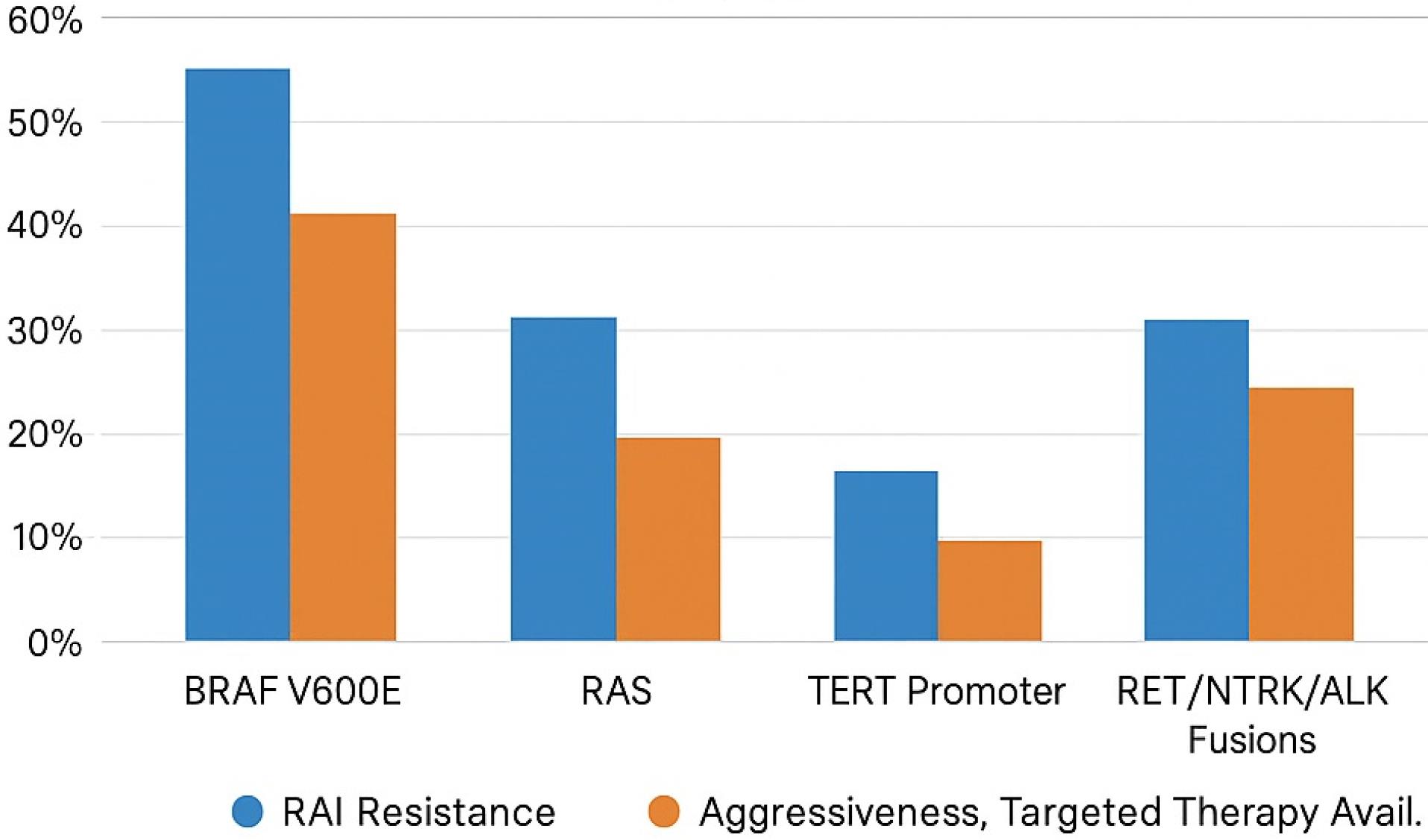
Figure 1.
Frequency and characteristics of genetic biomarkers in DTC
.
Frequency and characteristics of genetic biomarkers in DTC
Epigenetic biomarkers
DNA methylation
Hypermethylation in genes like RASSF1A, PTEN, and NIS is associated with de-differentiation, reduced iodine uptake, and bad prognosis in DTC.19,20 DNA hypermethylation in the CpG islands of the promoter regions causes transcriptional silencing of the tumor suppressor genes leading to de-differentiation and imparting radioiodine resistance.9,20 Recent studies found unique methylation patterns distinguishing between benign thyroid nodules and malignant DTC. This discovery could have significant implications for diagnosis, as such patterns might be used in point-of-care testing using sophisticated techniques like high-throughput sequencing and learning machines.9,19,20 Methylation-based markers are becoming popular in NGS, thus they can be included in comprehensive panels evaluating patients and determining the best therapeutic strategy.4,19
On the therapeutic front, demethylating agents like decitabine (5-aza-2’-deoxycytidine) are being studied to reverse the loss of RAI sensitivity. In early trials, it has been observed that decitabine acts by reactivating the silenced genes and thereby increasing iodine uptake, possibly leading to an improved therapeutic response. For instance, the reactivation of the NIS gene has been linked with an increase in iodine uptake and prolonged progression-free survival in some patients.19,20 Nevertheless, systemic toxicity is still a major hurdle, and the dosage-dependent side effects like myelosuppression underline the importance of using combination therapy to optimize efficacy.19,20 Newer generations of demethylating agents are currently being developed, which are more specific and less toxic. There are also studies going on to assess the potential of combination therapy that includes demethylating agents and histone deacetylase (HDAC) inhibitors in addition to immune checkpoint inhibitors, to further enhance the efficacy of the drug and overcome drug resistance. As our comprehension of DNA methylation progresses, these epigenetic modifications will likely assume a progressively crucial role in the stratification of risk and the decision-making for therapy in DTC.5,9,19,20
Histone modifications
HDAC inhibitors have come up as a very promising method for reversing the specific expression of thyroid genes, taking as example the symporter sodium-iodide (NIS), which is very important for the iodine absorption in the thyroid cells.21 HDACs remove the acetyl groups from the lysine residues on the histone tails. This eventually leads to the chromatin condensation and transcription of the tumor suppressor genes. HDAC inhibitors lead to the reverse of this process and induces the expression of the repressed genes involved in the processes of differentiation and iodine metabolism.21
Experiments are examining HDAC inhibitors (like vorinostat) combined with redifferentiation healing practices and displaying assurance in advancing NIS expression and RAI concentration. For instance, one phase II experiment combining vorinostat with retinoic acid showed elevated iodine accumulation in RAI-resisting DTC sufferers, indicating the potential of such a methodology to recover iodine appetency and enhance medical conclusions. Nonetheless, systemic toxicity continues to be a substantial hurdle, with potentially unfavorable side effects such as tiredness, gastrointestinal symptoms, and blood abnormalities serving as a reminder of the requirement for combination therapies to improve effectiveness.21
Research is underway to investigate better-targeted and less dangerous new HDAC inhibitors. There are studies on the effectiveness of HDAC inhibitors in conjunction with other drugs and immunotherapies to surmount resistance and increase performance. With the advancement of our knowledge regarding the histone modifications, these epigenetic changes are expected to be more significant in different treatment options and the degree of threat in differentiated thyroid cancer.21
Non-coding RNAs (ncRNAs)
Non-protein-coding RNAs, for example, miRNAs and long non-coding RNAs (lncRNAs), have significant activities in gene modifications of DTC. They are seen as regulatory factors in tumorigenesis, progression, and chemotherapeutic resistance in DTC, which can be used as diagnostic or prognostic markers.22
MicroRNAs (miRNAs)
MicroRNAs are small, non-coding RNA molecules (approximately 22 nucleotides long) that bind to the 3’-UTR of target mRNAs and regulate gene expression post-transcriptionally.22 For instance, miR-221, miR-222, and miR-146b have been demonstrated to be consistently up-regulated in aggressive DTC subtypes, with significantly poorer prognostic features, such as lymph node metastases, extrathyroidal extension, and radioiodine (RAI) refractoriness. These miRNAs can provide significant diagnostic and prognostic value, enabling the disease to be diagnosed more promptly and the risk to be more precisely classified. Antisense oligonucleotides (anti-miRs), which target oncogenic miRNAs, are currently being tested in preclinical studies, with the potential to restore cell differentiation and increase RAI uptake. In RAI-refractory DTC models, miR-221 inhibition, for example, has been shown to reverse tumor cell dedifferentiation and enhance radioiodine avidity.22
Long non-coding RNAs
Long non-coding RNAs (lncRNAs) are transcripts longer than 200 nucleotides that regulate gene expression at transcription, post-transcription, and at the epigenetic level, often acting as scaffolds for chromatin-modifying complexes.22 LncRNAs like HOTAIR and MALAT1 are overexpressed in aggressive DTC, correlating with tumor dedifferentiation, metastasis and poor survival. Inhibition of oncomodulators using RNA interference (RNAi) or CRISPR-based strategies is another promising therapeutic approach to suppress tumor growth and reverse RAI resistance. For example, downregulation of HOTAIR has been found to suppress proliferation and invasion in animal models providing insights into its potential as a therapeutic target.23
Nevertheless, the use of anti-ncRNAs in the clinical setting remains a challenge because of complications with delivery, durability and possible off-target effects.23 A variety of experiments are being conducted to develop new anti-miRs which are longer-lived and more targeted, as well as new delivery systems which use nanoparticles. Furthermore, various combinations of treatments are being examined so that ncRNA-targeting medications are partnered with immune checkpoint inhibitors and modulators of epigenetic changes so that the desired effect is strengthened. As the scientific knowledge of ncRNAs continues to evolve, the molecules will have an ever-increasing impact as it concerns the identification of patients at risk for developing DTC and the choice of treatment that will be most beneficial.23,24
Conclusion
Recent progress in molecular biomarkers has significantly impacted the diagnosis, prognosis, and treatment of DTC. Table 1 summarizes the key genetic and epigenetic biomarkers in DTC, including their clinical implications and therapeutic relevance. Genetic mutations, including BRAF V600E, RAS, and TERT promoter mutations, alongside epigenetic alterations and non-coding RNAs, offer valuable information about tumor behavior and response to therapy. These biomarkers have the potential to transform DTC management by facilitating earlier detection, accurate risk assessment, and personalized treatment strategies. For example, BRAF V600E testing is now a standard practice in preoperative risk assessment, while TERT promoter mutations help guide treatment decisions for aggressive cases.4
Table 1.
Key Genetic and Epigenetic Biomarkers in Differentiated Thyroid Cancer
|
Biomarker type
|
Name
|
Approx. frequency
|
Clinical features
|
Associated with RAI resistance
|
Targeted therapy available
|
| Genetic |
BRAF V600E |
40–60% |
Aggressive growth, metastasis, dedifferentiation |
Yes |
BRAF/MEK inhibitors (e.g., dabrafenib) |
| Genetic |
RAS (NRAS, HRAS, KRAS) |
10–40% |
Follicular pattern, moderate risk, potential dedifferentiation |
Moderate |
In development (e.g., MEK, PI3K inhibitors) |
| Genetic |
TERT Promoter |
10–20% |
Poor prognosis, distant metastasis |
Strongly associated |
Indirectly (under research) |
| Genetic |
RET/NTRK/ALK Fusions |
5–15% |
Common in young/post-radiation cases, targetable |
Variable |
FDA-approved (e.g., Retevmo, Vitrakvi) |
| Epigenetic |
DNA Methylation |
Not clearly defined |
Reduced differentiation, impaired iodine uptake |
Yes |
Demethylating agents (e.g., Decitabine) |
| Epigenetic |
Histone Modifications |
Not clearly defined |
Suppression of thyroid-specific gene expression |
Yes |
HDAC inhibitors (e.g., Vorinostat) |
| Non-coding RNA |
miRNAs / lncRNAs |
Under investigation |
Tumor progression, dedifferentiation, poor survival |
Yes |
Preclinical (e.g., anti-miRs, RNAi, CRISPR) |
The integration of molecular biomarkers into clinical practice has allowed for more personalized approaches to DTC treatment, particularly for patients with aggressive or RAI-refractory disease.4 Emerging technologies, such as liquid biopsy and NGS, further enhance risk stratification and treatment decision-making accuracy. These tools improve diagnostic accuracy and enable real-time monitoring of disease progression and treatment response, providing substantial benefits over traditional methods.6
Despite the potential advantages, challenges remain in implementing these biomarkers into routine clinical practice. Cost, accessibility, and the need for standardized guidelines must be addressed to ensure equitable access to these advancements.4 Further research is required to validate these biomarkers in diverse populations and integrate them into routine clinical practice.4 Liquid biopsy and NGS hold promise for improving outcomes in resource-limited settings, where access to advanced imaging and surgical expertise may be limited.6
As research continues to uncover new biomarkers and refine existing ones, the future of DTC management lies in the seamless integration of molecular data with clinical parameters to optimize patient outcomes while minimizing unnecessary treatment. These developments highlight the transformative potential of precision medicine in addressing the complexities of this heterogeneous disease. Moreover, advancements in DTC management serve as a model for precision medicine in other cancers, emphasizing the importance of integrating molecular data with clinical parameters to improve patient outcomes.4
Competing Interests
The authors of this article have no conflict of interest.
Ethical Approval
Not applicable.
Intelligence Use Disclosure
This article has not utilized artificial intelligence (AI) tools for research and manuscript development, as per the GAMER reporting guideline.
Acknowledgements
The authors would like to thank the officials of Shahid Beheshti University of Medical Sciences for their support of this project.
References
- Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev 2007; 28(7):742-62. doi: 10.1210/er.2007-0007 [Crossref] [ Google Scholar]
- Yu L, Hong H, Han J, Leng SX, Zhang H, Yan X. Comparison of survival and risk factors of differentiated thyroid cancer in the geriatric population. Front Oncol 2020; 10:42. doi: 10.3389/fonc.2020.00042 [Crossref] [ Google Scholar]
- Ma L, Guo H, Zhao Y, Liu Z, Wang C, Bu J. Liquid biopsy in cancer current: status, challenges and future prospects. Signal Transduct Target Ther 2024; 9(1):336. doi: 10.1038/s41392-024-02021-w [Crossref] [ Google Scholar]
- Kumar S, Gonzalez EA, Rameshwar P, Etchegaray JP. Non-coding RNAs as mediators of epigenetic changes in malignancies. Cancers (Basel) 2020; 12(12):3657. doi: 10.3390/cancers12123657 [Crossref] [ Google Scholar]
- Jiang S, Huang Y, Li Y, Gu Q, Jiang C, Tao X. Silencing FOXP2 reverses vemurafenib resistance in BRAF(V600E) mutant papillary thyroid cancer and melanoma cells. Endocrine 2023; 79(1):86-97. doi: 10.1007/s12020-022-03180-y [Crossref] [ Google Scholar]
- Hamidi S, Hofmann MC, Iyer PC, Cabanillas ME, Hu MI, Busaidy NL. Review article: new treatments for advanced differentiated thyroid cancers and potential mechanisms of drug resistance. Front Endocrinol (Lausanne) 2023; 14:1176731. doi: 10.3389/fendo.2023.1176731 [Crossref] [ Google Scholar]
- Lang M, Longerich T, Anamaterou C. Targeted therapy with vemurafenib in BRAF(V600E)-mutated anaplastic thyroid cancer. Thyroid Res 2023; 16(1):5. doi: 10.1186/s13044-023-00147-7 [Crossref] [ Google Scholar]
- San Román Gil M, Pozas J, Molina-Cerrillo J, Gómez J, Pian H, Pozas M. Current and future role of tyrosine kinases inhibition in thyroid cancer: from biology to therapy. Int J Mol Sci 2020; 21(14):4951. doi: 10.3390/ijms21144951 [Crossref] [ Google Scholar]
- Salvatore D, Santoro M, Schlumberger M. The importance of the RET gene in thyroid cancer and therapeutic implications. Nat Rev Endocrinol 2021; 17(5):296-306. doi: 10.1038/s41574-021-00470-9 [Crossref] [ Google Scholar]
- Li J, Yu Y. POU5F1B is responsible for the acquired resistance to dabrafenib in papillary thyroid cancer cells with the BRAF V600E mutation. Endocrine 2025; 87(1):220-33. doi: 10.1007/s12020-024-03994-y [Crossref] [ Google Scholar]
- Hu L, Zhang J, Tian M, Kang N, Xu G, Zhi J. Pharmacological inhibition of Ref-1 enhances the therapeutic sensitivity of papillary thyroid carcinoma to vemurafenib. Cell Death Dis 2022; 13(2):124. doi: 10.1038/s41419-022-04550-0 [Crossref] [ Google Scholar]
- Gao Y, Zhang D, Wang F, Zhang D, Li P, Wang K. BRAF V600E protect from cell death via inhibition of the mitochondrial permeability transition in papillary and anaplastic thyroid cancers. J Cell Mol Med 2022; 26(14):4048-60. doi: 10.1111/jcmm.17443 [Crossref] [ Google Scholar]
- Gild ML, Bullock M, Tsang V, Clifton-Bligh RJ, Robinson BG, Wirth LJ. Challenges and strategies to combat resistance mechanisms in thyroid cancer therapeutics. Thyroid 2023; 33(6):682-90. doi: 10.1089/thy.2022.0704 [Crossref] [ Google Scholar]
- Elia G, Patrizio A, Ragusa F, Paparo SR, Mazzi V, Balestri E. Molecular features of aggressive thyroid cancer. Front Oncol 2022; 12:1099280. doi: 10.3389/fonc.2022.1099280 [Crossref] [ Google Scholar]
- Silaghi H, Lozovanu V, Georgescu CE, Pop C, Nasui BA, Cătoi AF. State of the art in the current management and future directions of targeted therapy for differentiated thyroid cancer. Int J Mol Sci 2022; 23(7):3470. doi: 10.3390/ijms23073470 [Crossref] [ Google Scholar]
- Al-Jundi M, Thakur S, Gubbi S, Klubo-Gwiezdzinska J. Novel targeted therapies for metastatic thyroid cancer-a comprehensive review. Cancers (Basel) 2020; 12(8):2104. doi: 10.3390/cancers12082104 [Crossref] [ Google Scholar]
- Chen C, Liu J. Histone acetylation modifications: a potential targets for the diagnosis and treatment of papillary thyroid cancer. Front Oncol 2022; 12:1053618. doi: 10.3389/fonc.2022.1053618 [Crossref] [ Google Scholar]
- Gómez-Pérez AM, Cornejo Pareja IM, García Alemán J, Coín Aragüez L, Sebastián Ochoa A, Alcaide Torres J. New molecular biomarkers in differentiated thyroid carcinoma: Impact of miR-146, miR-221 and miR-222 levels in the evolution of the disease. Clin Endocrinol (Oxf) 2019; 91(1):187-94. doi: 10.1111/cen.13972 [Crossref] [ Google Scholar]
- Liu Y, Wang J, Hu X, Pan Z, Xu T, Xu J. Radioiodine therapy in advanced differentiated thyroid cancer: Resistance and overcoming strategy. Drug Resist Updat 2023; 68:100939. doi: 10.1016/j.drup.2023.100939 [Crossref] [ Google Scholar]
- Tuttle RM, Alzahrani AS. Risk stratification in differentiated thyroid cancer: from detection to final follow-up. J Clin Endocrinol Metab 2019; 104(9):4087-100. doi: 10.1210/jc.2019-00177 [Crossref] [ Google Scholar]
- Jia M, Liang J, Gao L, Wei N, Qin Y, Li Q. Navigating thyroid cancer complexity: the emerging role of EV-derived non-coding RNAs. Cell Death Discov 2025; 11(1):142. doi: 10.1038/s41420-025-02411-1 [Crossref] [ Google Scholar]
- Sheikholeslami S, Azizi F, Ghasemi A, Alibakhshi A, Parsa H, Shivaee S. The epigenetic modification of SLC5A8 in papillary thyroid carcinoma and its effects on clinic-pathological features. Iran J Public Health 2022; 51(3):634-42. doi: 10.18502/ijph.v51i3.8940 [Crossref] [ Google Scholar]
- Zarkesh M, Zadeh-Vakili A, Azizi F, Foroughi F, Akhavan MM, Hedayati M. Altered epigenetic mechanisms in thyroid cancer subtypes. Mol Diagn Ther 2018; 22(1):41-56. doi: 10.1007/s40291-017-0303-y [Crossref] [ Google Scholar]
- Yu X, Zhang H, Zhang H, Hou C, Wang X, Gu P. The role of epigenetic methylations in thyroid cancer. World J Surg Oncol 2024; 22(1):281. doi: 10.1186/s12957-024-03568-2 [Crossref] [ Google Scholar]