Biomed. adv. 1(1):2-11.
doi: 10.34172/bma.02
Review Article
Regenerative medicine and congenital heart diseases: Current approaches and future directions
Buse Sanli Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, 
Mohammadreza Dastouri Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – review & editing, * 
Author information:
Department of Medical Biology and Genetics, Faculty of Medicine, Ankara Medipol University, Eti, Celal Bayar, Cankaya / Ankara, Turkey
Abstract
Summary
Congenital heart disease (CHD) is one of the most common disorders in newborns. The disease may be present in as many as 0.013% of newborns. Even with the vast development of the medical field and surgical interventions, the fact remains that serious CHDs are the leading cause of death and disability for children who may have to endure long-term problems such as heart failure and other systemic issues. About 80% of infants and toddlers with CHDs have the potential to reach adulthood. Hence, the cost and effectiveness of care for such patients have become complex. Advances in regenerative medicine, specifically stem cell therapies, have significantly progressed in treating CHD. Different stem cells were used together with the corresponding findings, with success, in the separate preclinical works. These methods are planned to treat heart problems, thus reducing complications and improving the heart’s overall function. However, drawbacks like immune rejection, scalability, and safety hinder the application of these approaches to clinical practice. This paper comprehensively explores the existing CHD management techniques in regenerative medicine, including tissue engineering, gene therapy, and biomaterials development. Furthermore, issues such as 3D bioprinting, CRISPR gene editing, and the use of organoids for heart tissue modeling are also discussed. Steady progress has been realized in treating these diseases using stem cells. However, ethical considerations and legislation on the use of stem cells, in general, should become a part of the study and review process for already safe and fair patient access. The key to curing CHD diseases will be to overcome the challenges and to bring preclinical successes to life in the clinic. By enhancing our knowledge about heart formation and refinement of regenerative treatments, we would have more success stories with CHD patients and help them obtain a better quality of life and a brighter future.
Keywords: Congenital heart disease, Regenerative medicine, Clinical trials, Cell therapy
Copyright and License Information
© 2024 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
None.
Introduction
Congenital heart disease (CHD) is a structural abnormality of the heart that develops during fetal development. It is the most common congenital malformation in infants, with a prevalence of 0.006 to 0.013% of live births.1-3 There have been many advances in medical care and surgical interventions for infants with CHD. Still, the mortality and morbidity associated with severe forms of the disease remain high, making it one of the leading causes of death among congenital anomalies. In the past decade, the clinical focus of CHDs has shifted toward adult care, as recent estimates indicate that approximately 80% of infants and toddlers with CHDs now have the potential to reach adulthood. These patients often face complications such as heart dysfunction, heart failure, and respiratory, nervous, and coagulation system problems. The cost of treating these diseases is very high and imposes much financial pressure on the families of the patients and the countries’ medical systems. Using stem cells in treating heart diseases has created great hope among scientists for using these cells in CHD. Many challenges exist in regenerative medicine, including immune rejection and cell matching. However, different stem cells, such as embryonic stem cells (ESCs), adult stem cells, induced pluripotent stem cells (iPSCs), and mesenchymal cells (MSCs), have been used in much research, and valuable results have been obtained. In this review, we will study various stem cell therapies that have recently been applied to CHDs and discuss future directions for stem cell-based therapies and the field of regenerative medicine for the effective management of CHDs.
Present strategies in regenerative medicine for congenital heart conditions
CHD refers to a variety of structural heart anomalies that are present at birth and are the result of disruptions in fetal heart development. The most frequent defects include atrial septal defects, which are also known as atrial septal defects, and more complex anomalies such as hypoplastic left heart syndrome (HLHS).4 CHD is the most significant congenital disability worldwide, affecting millions of newborns annually.5 The most prevalent treatments for CHD are traditional surgeries, catheter-based techniques as well as drug therapy. On the other hand, surgeries like the reconstruction of valves are used in many cases. Sometimes, the patient must undergo other operations later in life.6
One of the newer methods is catheter interventions, which include deploying stents or occluders in blood vessels. These interventions are minimally invasive and sometimes used.7 Medications manage symptoms and improve heart function but do not address the underlying structural issues and have troublesome side effects.8 Even though traditional methods for treating the condition have apparent advantages and give patients more time to live and experience a better quality of life, there is still no definitive treatment for them. As a result, some of them are struggling with side effects and are in danger of the ailment worsening. For this reason, researchers have been looking for solutions to treat this disease in recent years. Along with advances in stem cells, gene editing technology, and organoids, many hopes have been made for alternative treatments for these diseases. These developments can be classified as follows:
Stem cell therapies
Stem cell therapies have emerged as a promising area of regenerative medicine for dealing with CHD, amongst other tissue or heart issues. Nevertheless, the possibility of fixing a heart destroyed by disease or injury illustrates the potential of stem cell-based therapies. As opposed to traditional treatments that only suppress the symptoms or necessitate surgical correction of structural faults, stem cell approaches pave the way to bring back the perfection of the heart by growing the wings once afflicted areas.9
ESCs, iPSCs, and MSCs are among the stem cells studied for their capability to differentiate into cardiomyocytes and endothelial progenitor cells.10,11 Preclinical studies have shown that injected stem cells can form a union with the heart tissue, generate the blood supply, and improve the heart’s functionality in CHD models.12
One of the significant challenges of successfully implementing stem cell therapies is ensuring the secure and effective differentiation and integration processes of cells into heart tissue.13 Breakthroughs in biomaterials and tissue engineering have explained the advancement of new methods for the survival and functionality of transplant stem cells, like scaffolds or injectable hydrogels that can simulate the heart’s natural extracellular matrix.14 Similarly, a study transduces genes into these cells to increase their regenerative competence or lessen the risk of immune rejection. While appearing to be just in the infant stages, future clinical trials of stem cell therapy indicate new hope for treating these patients, decreasing the need for repeat surgeries and significantly increasing their long-term survival.15 Regenerative medicine strategies for treating CHD are shown in Figure 1.
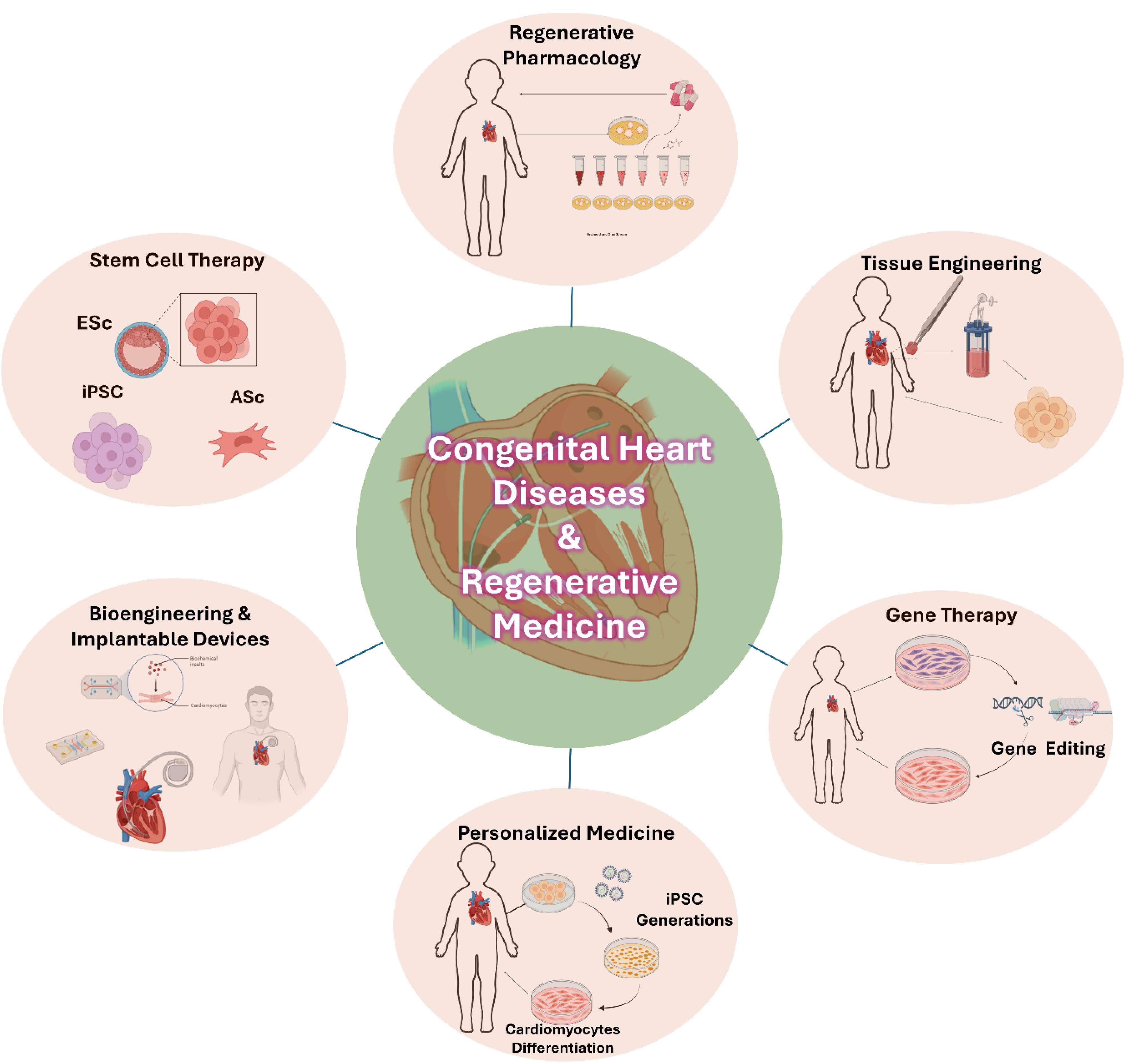
Figure 1.
Regenerative medicine in congenital heart disease
.
Regenerative medicine in congenital heart disease
Tissue engineering techniques
Tissue engineering has become crucial in treating CHD, offering innovative solutions to reconstruct or replace damaged heart tissues. Unlike traditional surgical approaches, tissue engineering utilizes a combination of biomaterials, cells, and bioactive molecules to create functional heart tissues that can grow and adapt to the patient (Figure 1).16
One of the main methods this occurs is through biodegradable scaffolds with autologous or allogeneic cells supported by new heart muscle regeneration. As the scaffolds biodegrade, they are no longer detectable and are replaced by regenerated tissue.16-19
Structural scaffolds are commonly engineered to mimic the heart’s extracellular matrix, offering the essential mechanical reinforcement and biochemical signals required for tissue regeneration.19 Recent breakthrough developments in three-dimensional (3D) printing have made bioengineering for cardiac diseases significantly more perfect. This procedure allows the precise production of complex cardiac tissues through the placement and layering of cells and biomaterials. It is possible to make heart tissues, even complete heart valves.20,21 In addition to these new technologies, decellularized tissues, natural tissues isolated from their cells, are also suitable materials for heart tissue engineering. These matrices keep the heart’s natural architecture and mechanical properties intact, which sustains a very appropriate environment for cell proliferation and tissue regeneration.22 Additionally, incorporating stem cells into engineered tissues has also benefited regeneration. Animal studies have tried using stem cell-seeded scaffolds, and the results were that they might help with vascularization and myocardial function.23
Gene therapy innovations
Gene therapy is promising for patients with genetic diseases, especially cardiovascular diseases with genetic factors, because some cardiovascular diseases are hereditary. Gene therapy has opened a new window for the treatment of heart diseases, including CHD. So far, various methods have been used in gene therapy. However, with the development of CRISPR/Cas9 technology in recent years, genetic editing can be done more efficiently and practically, providing a new possibility for treating CHD patients. Advances in the treatment of some CHD can allow the permanent treatment of some causes. Moreover, correcting genetic abnormalities during fetal development with this treatment method before birth is possible. As a result, some CHD can be prevented. In recent years, much research has been done in treating these diseases by relying on gene therapy methods at the cellular level and animal models, such as gene therapy research on DiGeorge syndrome,24 Barth syndrome,25 Wolff-Parkinson-White disease syndrome,26 Duchenne muscular dystrophy,27 Holt-Oram syndrome,28 Heterotaxy syndrome,29,30 Noonan syndrome,31 Marfan syndrome,32,33 and nonsyndromic,34,35 which has been done at the cellular level and in animal models. Generally, relatively promising results have been obtained.36
While favorable results have been obtained from the research on gene therapy in treating heart diseases, the clinical use of gene therapy still faces many obstacles, ethical problems, and side effects. This treatment method must be solved before it can be used clinically to treat these diseases.
Preclinical and clinical studies and results
Preclinical studies are required to investigate new therapies in the field of CHD. They are preferred for their rapid results, cost-effectiveness, and capability to efficiently reach sufficient groups and subjects.37 In the following, we will mention some of the research.
Preclinical studies
Right ventricle dysfunction-induced neonatal porcine models used by Wehman et al for MSC infusion. In the treated group, neovascularization and myocardial strain mechanics are improved. Cardiomyocyte and endothelial cell proliferation potential increased. Moreover, right ventricular thickness remains stable in the treated group rather than the placebo group’s increasing thickness. This result demonstrated that MSC therapy suppresses hypertrophy, which is an impact of right ventricle dysfunction.38
MSC-seeded bioscaffolds are used on pulmonary arterial hypertension-induced rats to improve right ventricle function. Results showed that bioscaffolds increased right ventricle function. Additionally, although RV afterload was increased, the right ventricle could maintain its contractility. Also, treatment decreased collagen accumulation, cardiomyocyte hypertrophy, and right ventricle fibrosis.39
Trac et al studied efficacy differences in 3D and 2D transfers of cardiac progenitor cells. It is observed that the implantation of 3D CPC is more efficient for improving right ventricular function than 2D implantation on athymic and right ventricle failure-induced rats. Moreover, according to the results, 3D implantation of CPC reduced fibrosis and hypertrophy and promoted angiogenesis.40
In 2019, a preclinical study used graft cellularization technology to regulate the right ventricular outflow tract (RVOT). The viability of grafts from thymus mesenchymal stem cells (T-MSCs) isolated from pig thymus and ECM derived from the small intestine submucosa was studied. The results demonstrated reduced fibrosis, improved RVOT contractility, endothelialization, and cardiac remodeling were observed, and the application’s safety and feasibility were verified 41.
In 2019, human neonatal thymus mesenchymal stem cells’ (NTMSCs’) effects on SOD3 secretion were researched. SOD3 is an enzyme secreted by the left ventricle in response to reducing the impacts of right ventricle failure. At the end of the research, it was observed that NTMSCs have higher transcription expression than other cells. The same results were also examined in 3D cell culture. Moreover, it has emerged that cell sheet culture is a better stimulator for SOD3 than monolayer culture. According to the results, NTMSCs are one of the best choices for cell therapy.42
Nana-Leventaki et al studied the infusion of cardiosphere-derived cells (CDCs) in autoimmune myocarditis-induced rats. Results showed that infusion is efficient in terms of left ventricular ejection fraction (LVEF) and LV end-systolic volume. Additionally, CDC infusion reduced myocardial inflammation, fibrosis, and T-cell infiltration.43
Limitations of preclinical studies
Animals used in studies have specific anatomical differences with humans. They also tend to react differently to treatments, negatively affecting the study. Additionally, the cost of animals in extensive studies limits the number of animals used in research 37.
Clinical trials (patient outcomes and long-term impact)
Clinical trials are critical in translating research for CHDs from the bench to the bedside. These trials explore interventions like stem cell treatment, tissue regeneration, and genetic modification to identify their effectiveness, safety profile, and possible benefits compared to existing treatments for CHD. Even though these are not new findings, they play an essential role in creating new therapies for patients suffering from this multifaceted disorder, which can improve their quality of life.
In the Transcoronary Infusion of Cardiac Progenitor Cells in Patients With Single-Ventricle Physiology (TICAP) clinical trial, HLHS patients were observed for 36 months after the intracoronary infusion of CDC. The absence of infections or tumor formation, the maintenance of tumor marker values at normal levels, and the absence of myocardial ischemia or arrhythmia were significant findings. The cardiac magnetic resonance imaging (CMRI) test results showed improved prop right ventricle ejection fraction (RVEF) values in the treatment group compared to the control group. The New York University Pediatric Heart Failure Index scores were also lower in the treatment group. Researchers highlighted the significant role of youthfulness in improving RVEF values, offering an optimistic outlook for future research in this area 44.
In another clinical trial, an autologous injection of cord blood mononuclear cells (CBMNCs) after Norwood’s heart operation indicated that the infusion of CBMNC was safe and feasible. This method is one of the earliest interventions for developing the right ventricle when adaptation capacity is at the highest level. Early intervention can help the patient navigate the critical period more efficiently. Infusion also helps stimulate the myocardium with paracrine effects. Three mortality rates were reported during the trial, but none were related to cell therapy. Five treated patients’ results were compared to 22 similar cases. CBMNC treatment applied patients’ RVEF and RVSVi (right ventricle stroke volume index) values were higher.45 Some clinical trials with patient outcomes and long-term impact research are summarized in Table 1.
Table 1.
Some of the completed clinical trials for the treatment of CHD with stem cell-based therapy
|
Phase
|
Cell type, injection, dose
|
Disease
|
Participant number
|
Results
|
Research group
|
| Phase I |
Autologous cardiac progenitor cells intracoronary infusion 0.3 million/kg |
HLHS |
14 |
RVEF improved brain natriuretic peptide levels, decreased z score, improved need for unplanned catheter interventions, decreased |
Tarui et al44 |
| Phase I |
Autologous cord blood stem cell coronary infusion 1.5 ± 1.1 × 106 CBSCs |
HLHS patients within 2-3 days of birth |
10 |
RVEF increased RVSVi increased RVMI with no difference |
Brizard et al45 |
| Phase I |
Autologous umbilical cord blood cells intramyocardial injections 3 million cells per kilogram |
HLHS at the time of stage II surgical palliation |
30 |
The procedure was safe and feasible |
Burkhart et al46 |
| Phase II |
Autologous cardiosphere-derived cells intracoronary infusion 3.0 × 105/kg |
Univentricular heart disease |
34 |
ventricular volumes and fibrosis decreased heart failure status, and somatic growth improved |
Ishigami et al47 |
A clinical trial completed in 2021 used umbilical cord blood (UCB) derived products different from those provided by UCB banks, which contain high-value mononuclear cell products and allow direct application use (NCT01856049). Patients injected with the product fulfilled the criteria, and it was verified that the product was safe for use. In 10 patients who participated in the research, Stage II Bi-directional Cavo-pulmonary Connection (BCPC) and intramyocardial (UCB-MNC) infusion were implemented. There was one reported mortality. However, it was not relevant to cell therapy. No arrhythmia, myocardial ischemia, or hemodynamic instability was observed in patients. Results indicated that the UCB-MNC application is safe and feasible.46 A study that focuses on univentricular heart diseases has two objectives. The first objective is to evaluate the effectiveness of autologous CDC infusion with a 3-month follow-up after the palliation. The second objective is to observe the effects of infusion on all participants (including those requesting the injection after the first three months) during the 12-month follow-up. At the end of 3 months, CDC infused patients’ ventricular ejection fraction (VEF) values, ventricular volume, and strain ratio improved. Even in the patients who joined later, progress was made. After 12 months, similar improvements in recovery were observed. After 3 and 12 months of follow-up, the New York University Pediatric Heart Failure Index score was significantly increased in treated patients. Additionally, somatic growth retardation and z-score results were enhanced. Transient troponin-I and -T levels were found in the treatment group, but the findings returned to baseline by the third day of infusion.47 Some of the recruiting/ active/ terminated clinical trials for the treatment of CHD with stem cell-based therapy were summarized in Table 2.
Table 2.
Some of the recruiting/active/terminated clinical trials for the treatment of CHD with stem cell-based therapy
|
Phase
|
Cell type, Injection
|
Disease
|
Participant number
|
Status
|
Trial ID
|
| Phase III |
Autologous cardiac stem cell intracoronary injection |
After reconstructive surgery in pediatric patients with functional single ventricle |
40 |
Recruiting |
NCT02781922 |
| Phase I |
Autologous UCB-MNC intramyocardial injections |
Single right ventricular dependent circulatory systems define severe CHD |
30 |
Active |
NCT04907526 |
| Phase I |
Autologous IPSCL |
Univentricular CHD |
50 |
Recruiting |
NCT05647213 |
| Phase I |
Allogeneic mesenchymal stem cells intramyocardial injection |
HLHS |
30 |
Terminated |
NCT02398604 |
| Phase I |
Autologous cardiac stem cell (c-kit + ) intramyocardial injection |
HLHS |
32 |
Recruiting |
NCT03406884 |
| Phase I |
Allogeneic Lomecel-B (formerly LMSCs) intramyocardial injections |
HLHS |
10 |
Active |
NCT03525418 |
| Phase II |
Allogeneic Lomecel-B intramyocardial injections |
HLHS |
38 |
Recruiting |
NCT04925024 |
| Phase I-II |
Allogenic mesenchymal precursor cells injection into the LV endocardium |
HLHS, unbalanced atrioventricular canal (uAVC), or borderline left heart |
19 |
Active |
NCT03079401 |
| Phase IIb |
Autologous umbilical cord blood-derived mononuclear cells ıntramyocardial ınjection |
HLHS |
95 |
Active |
NCT03779711 |
Barriers to implementing research in clinical settings
Generally, long-term patient follow-up is not applied, so treatments’ future impacts remain unclear. The need for more current applications restricts the ability to achieve definite results. Also, the absence of volunteered patients and, in some cases, the lack of a placebo group in the study may affect the results undesirable
Cutting-edge techniques and emerging innovations
Progress in 3D bioprinting
Current advancements in injured tissue treatments are limited in terms of accessibility and feasibility; therefore, there is a need for novel approaches. Bioprinting technology provides an effective alternative to current methods. Bioprinting is utilized to generate anatomically corrected biological structures; it is an important part of tissue engineering. In 2019, research addressing the use of personalized hydrogels presented two models. The first model contains iPSC-derived cardiomyocytes and endothelial cells for patient-specific treatment. The second model contains neonatal mouse cardiomyocytes, human umbilical vein endothelial cells, and lumen-supporting fibroblasts. To generate a 3D cardiac patch, a patient’s tomography was performed to obtain anatomical information. Afterward, personalized hydrogels were infused with iPSC-derived cardiomyocytes. The function of the printed vascularized cardiac patch was evaluated by testing calcium transients to control the patches, and it was transplanted into a rat. As a result, the cells extended, aligned, and exhibited potential for contractility. This study demonstrated that bioprinting technology is safe and feasible for generating 3D cardiac patches with the patient’s cells.48
Advancements in CRISPR/Cas9 and genetic engineering
Understanding the mechanism and how it occurs can help treat a disease. CRISPR/Cas9 technology allows researchers to identify variants and diagnose diseases. This technology can also be an alternative to creating a model for researching and curing diseases.49 Most genes affecting CHDs are also related to cardiac cell differentiation. Therefore, identifying genetic variants is essential in treating these diseases, which often have different genetic variants.
Heart model cells generated from iPSCs with the CRISPR/Cas9 gene editing technique were used to study unknown effects. The advancements in CRISPR/Cas9 homology-directed repair technology allow human models to be used for research. The research focuses on the uncertain variants of transcription factor GATA4, which effectively differentiates cardiomyocytes and has pathogenic variants that impair heart maturation. GATA4 variants with uncertain effects (GATA VUS) were transferred into IPSCs using the CRISPR/Cas9 gene editing technique, and these cells differentiated into cardiomyocytes. The exact process was applied using healthy GATA4 cardiomyocytes (GATA4 WT), and the resulting cells were compared against various criteria with GATA4 VUS cells to determine whether they were consistent with the patient’s phenotype. A GATA4 genetic variant that was not compatible with the control group was found during the research. This variant is classified as a variant with unknown effects. The effects of these variants on cardiomyocyte differentiation were investigated. GATA4 interacts functionally with a cofactor, and this cofactor shows reduced interactions with insignificant variants. Therefore, observing the expression of the variant in tissue could provide a better result. The variant was transfected into IPSCs using CRISPR/Cas9 technology to form a cardiac model. In the screening result, the presence of transfected alleles was verified. This finding indicates the viability of CRISPR/Cas9 in IPSC clones. GATA WT and the newly identified variant’s cardiomyocytes were compared in terms of heart rate, GATA4 localization, and cardiac cell expression. In the results of the identified variant’s cardiomyocytes, irregular contractions and reduced excitability related to calcium regulation were observed, consistent with the patient’s phenotype. This research demonstrated CRISPR/Cas9 technology’s effectiveness in determining various variant impacts and provided a model for CHD genetic variants.50
Development of organoids and lab-cultivated heart tissue
Understanding the heart’s anatomical structure and physiology is essential for treating CHD. For this reason, studying the heart’s development plays a significant role in understanding these features. During the heart’s growth in the cardiac crescent, two different populations of cardiac progenitor cells differ from each other based on the time they contributed to the cardiac lineage. These populations form the first and second heart fields. In 2021, a rat model study showed the differences between these two structures and provided information about heart morphogenesis. The effects of precardiac mesoderm on cardiac proliferation and differentiation were investigated. Additional evidence was found that Wnt signals provided by cardiac progenitors play a role in determining the cell fate of second heart fields. In this regard, studying the mechanism that enables cardiac progenitor cell differentiation, proliferation, and maturation is crucial for understanding the reasons for heart defects.51
Small molecule therapy
Small molecule therapy is a promising approach for treating CHD that targets critical molecular pathways involved in cardiac growth and repair. Small molecules are compounds with low molecular weight; therefore, they can pass very easily through the cell membranes and be the biological substances that change the status of proteins, such as the interactions between the enzyme activity and the cell signaling pathways.52 For instance, in a CHD situation, they can direct the operation of such processes as angiogenesis, fibrosis, and cardiac remodeling to increase the heart’s functionality and reduce the disease burden. Recent advances in understanding the molecular underpinnings of CHD have opened the way for small-molecule interventions.
Zhang et al studied cardiomyocyte maturation using the single-cell RNA-Seq (scRNA-Seq) technique on postnatal rat hearts. Most adult mammalian cardiomyocytes are deficient in their ability to proliferate. This leads to studies on how cardiomyocytes lose their proliferation ability, affecting their regeneration capacity. It was observed that the proliferation capacity decreased proportionally with maturation. The adult mammalian heart is polyploid and non-proliferative. In contrast, the rat heart can proliferate within the first week after birth. After this, the rat’s heart develops polyploidy and loses its ability to regenerate. It was observed that the number of mononuclear diploid cardiomyocytes in rat hearts is significant for heart regeneration after injury. Therefore, investigating the mammalian heart is essential for identifying the factors that initiate regeneration.
The AP-1 transcription factor involves numerous cellular activities (apoptosis, differentiation, division). Additionally, it has been observed that this protein promotes heart regeneration following injury in zebrafish. However, its effects on cardiomyocyte maturation remain unknown. To investigate the cardiomyocyte maturation mechanism, the study used scRNA-Seq on neonatal rat ventricle cells collected on the first and seventh days after birth. Using the scRNA-Seq technique in human cardiomyocytes, it was demonstrated that the AP-1 protein family was temporarily increased and activated during the 22nd week of embryonic development. Therefore, it is reasonable to consider that these proteins may be effective in cardiomyocyte maturation.
Moreover, when adult rat cardiomyocytes are analyzed regarding several nuclei, observations indicate that the number of nuclei is related to the AP-1 protein family. When comparing 4n human IPSCs, 2n cardiomyocytes have more AP-1 binding motifs. The results have displayed that the AP-protein family has become activated in maturation and may play a role in this mechanism. In further study sections, one of the AP-1 proteins suppresses cytokinesis and promotes and regulates maturation in cardiomyocytes. Understanding cardiomyocyte maturation mechanisms represents a significant advancement in treating congenital diseases. The data suggest that the AP-1 protein family could be utilized to treat CHD.53
Challenges and limitations
-
Complexity of heart structure and function:Because of its intricate nature, the heart is challenging to recreate or treat regenerative medicine. Many different types of CHD require complicated and precise interventions.
-
Immune rejection and compatibility: Many immunological challenges exist when stem cells or engineered tissues are introduced into patients. Immunological rejection of foreign tissues/cells still poses significant challenges in regenerative medicine.
-
Scalability and production issues: One of the biggest challenges is coping with clinical demand by increasing the production of engineered tissues or stem cells. The production process must be consistent, reproducible, and able to be transferred on a larger scale.
-
Long-term efficacy and safety: Ensuring the long-term survival, integration, and functioning of transplanted cells or tissues is essential. Risks might include tumorigenesis, loss of function over time, or even arrhythmias.
Ethical and regulatory issues
Ethical concerns related to stem cell use
New ethical worries are emerging about the growth and application of cells, such as stem cells, which can turn into different kinds of human tissue. As stem cell studies advance to clinical uses, expect to see more ethical questions emerge. These will focus on translating basic stem cell research into patient treatments.54
To change the direction of bioethics talks from embryo status to more comprehensive stem cell uses, must focus on values we share, such as keeping patients safe, doing ethical research, and promoting fairness in society. As we see more diagnoses and treatments based on stem cells, people worry about too much profit-seeking and tight patents, which might stop many from getting the benefits of this research. Making sure the public gains from this work gives us a strong moral reason to do stem cell research. As we test these treatments on people, we must deal with social fairness issues head-on by rethinking how we handle patents and funding so everyone can access stem cell therapies. We should also get more input from community members and patient supporters to oversee the rules. Tackling these tricky bioethics questions is critical as researchers worldwide push ahead with stem cell studies.54,55
Regulatory hurdles
Strict regulatory requirements must be applied to develop and approve regenerative therapies. These include safety assessments, clinical trials, and compliance with international standards, which can delay the introduction of new treatments. The aim is to regularly notify the scientific community, healthcare stakeholders, and patient groups about approved products to intensify dialogue around highly anticipated and recognized therapeutic strategy.56 Furthermore, patients’ and public perceptions must be considered when developing treatments that will subsequently shape their uptake. Accurate information has to reach patients and their caregivers to make them understand issues related to perceptions toward acceptance of cell and gene therapy. Before getting involved in clinical trials or accessing licensed regular treatment, patients must know the possible advantages of this approach, as well as its disadvantages.57
Informed consent and patient autonomy
Research in regenerative therapies that require informed consent has its intricacies, especially when there are people from the vulnerable, like kids suffering from CHD. This means that guardians and their parents should understand the dangers and advantages of such practices before proceeding.58
Future directions in regenerative medicine for congenital heart diseases
Stem cell-based therapies show that the heart tissue is regenerated, specifically using different types of pluripotent and adult stem cells. In this way, individual cells can differentiate into functional cardiac cells of various types, producing a line of heart tissue repair or replacement in CHD patients with defective heart tissues.
However, issues like immune rejection, cell compatibility, and scalability linked to the extensive incorporation of the technology should be addressed. Stem cell therapy treatments and other tissue engineering and gene editing technologies, such as CRISPR/Cas9, are predicted to treat CHD patients. The technology of 3D bioprinting is the way for it to create patient-specific cardiac tissues and patches through the printer. Also, these advanced medical techniques allow the production of organoids and heart tissue in a lab, which is both the disease model for CHDs and the trial ground for other drugs.
Regulations related to social and ethical issues should be considered. Additionally, combining modern methods of genetic engineering and bioprinting with traditional treatments for heart diseases is likely a solution that will allow the evolution of current healthcare and treatment systems.
Furthermore, while investigation and evaluation for the safety and success of these novel treatments over time are significant issues, stem cell utilization, and genetic modification are critical components of this topic. The research will answer these challenges and spearhead the field to focus on treatment that enables the development of these vital technologies in patients.59,60
Conclusion
Regenerative medicine has the potential to be one of the best treatment methods for CHD; it offers possible answers that are unattainable in traditional medicine and surgery practice. With advancements made possible through stem-cell therapy, tissue engineering, and genetic engineering, avenues have been opened that would lead to developing more effective treatments for CHD patients, which could change the quality of life and decrease high morbidity and mortality rates.
Nonetheless, significant barriers are associated with the widespread clinical application of these therapies, including intricacies of the heart’s structure/function, immunological rejection issues, scalability problems, and chronic effectiveness. As worries about ethical standards in biology increase, we can’t ignore the moral questions associated with using stem cells. Such ethical issues require thoughtful handling to prevent unequal distribution and limited access to treatments in regenerative medicine.
Future research should focus on overcoming these challenges by improving current technologies, developing new treatments, and addressing concerns surrounding ethical and regulatory issues. Achieving this goal will pave the way for a new generation of regenerative medicine therapies for CHD, ultimately leading to a brighter and healthier future for patients globally.
Competing Interests
The authors declare no conflicts of interest.
Ethical Approval
Not applicable.
References
- Ibrahim S, Gaborit B, Lenoir M, Collod-Beroud G, Stefanovic S. Maternal pre-existing diabetes: a non-inherited risk factor for congenital cardiopathies. Int J Mol Sci 2023; 24(22):16258. doi: 10.3390/ijms242216258 [Crossref] [ Google Scholar]
- Dilli D, Akduman H, Zenciroğlu A, Çetinkaya M, Okur N, Turan Ö. Neonatal outcomes of critical congenital heart defects: a multicenter epidemiological study of Turkish Neonatal Society: neonatal outcomes of CCHD. Pediatr Cardiol 2024; 45(2):257-71. doi: 10.1007/s00246-023-03362-z [Crossref] [ Google Scholar]
- Hossin MZ, de la Cruz LF, McKay KA, Oberlander TF, Sandström A, Razaz N. Association of pre-existing maternal cardiovascular diseases with neurodevelopmental disorders in offspring: a cohort study in Sweden and British Columbia, Canada. Int J Epidemiol 2024; 53(1):dyad184. doi: 10.1093/ije/dyad184 [Crossref] [ Google Scholar]
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002; 39(12):1890-900. doi: 10.1016/s0735-1097(02)01886-7 [Crossref] [ Google Scholar]
- van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol 2011; 58(21):2241-7. doi: 10.1016/j.jacc.2011.08.025 [Crossref] [ Google Scholar]
- Holst KA, Said SM, Nelson TJ, Cannon BC, Dearani JA. Current interventional and surgical management of congenital heart disease: specific focus on valvular disease and cardiac arrhythmias. Circ Res 2017; 120(6):1027-44. doi: 10.1161/circresaha.117.309186 [Crossref] [ Google Scholar]
- Rome JJ. The role of catheter-directed therapies in the treatment of congenital heart disease. Annu Rev Med 1995; 46:159-68. doi: 10.1146/annurev.med.46.1.159 [Crossref] [ Google Scholar]
- Varela-Chinchilla CD, Sánchez-Mejía DE, Trinidad-Calderón PA. Congenital heart disease: the state-of-the-art on its pharmacological therapeutics. J Cardiovasc Dev Dis 2022; 9(7):201. doi: 10.3390/jcdd9070201 [Crossref] [ Google Scholar]
- Tsilimigras DI, Oikonomou EK, Moris D, Schizas D, Economopoulos KP, Mylonas KS. Stem cell therapy for congenital heart disease: a systematic review. Circulation 2017; 136(24):2373-85. doi: 10.1161/circulationaha.117.029607 [Crossref] [ Google Scholar]
- Afjeh-Dana E, Naserzadeh P, Moradi E, Hosseini N, Seifalian AM, Ashtari B. Stem cell differentiation into cardiomyocytes: current methods and emerging approaches. Stem Cell Rev Rep 2022; 18(8):2566-92. doi: 10.1007/s12015-021-10280-1 [Crossref] [ Google Scholar]
- Dastouri M, Ozdag H, Akar AR, Can A. Differentiation and molecular characterization of endothelial progenitor and vascular smooth muscle cells from induced pluripotent stem cells. Bioimpacts 2023; 13(4):289-300. doi: 10.34172/bi.2022.24132 [Crossref] [ Google Scholar]
- Rao KS, Kameswaran V, Bruneau BG. Modeling congenital heart disease: lessons from mice, hPSC-based models, and organoids. Genes Dev 2022; 36(11-12):652-63. doi: 10.1101/gad.349678.122 [Crossref] [ Google Scholar]
- du Pré BC, Doevendans PA, van Laake LW. Stem cells for cardiac repair: an introduction. J Geriatr Cardiol 2013; 10(2):186-97. doi: 10.3969/j.issn.1671-5411.2013.02.003 [Crossref] [ Google Scholar]
- Chen MH, Wang LL, Chung JJ, Kim YH, Atluri P, Burdick JA. Methods to assess shear-thinning hydrogels for application as injectable biomaterials. ACS Biomater Sci Eng 2017; 3(12):3146-60. doi: 10.1021/acsbiomaterials.7b00734 [Crossref] [ Google Scholar]
- Phillips MI, Tang YL. Genetic modification of stem cells for transplantation. Adv Drug Deliv Rev 2008; 60(2):160-72. doi: 10.1016/j.addr.2007.08.035 [Crossref] [ Google Scholar]
- Harris AG, Salih T, Ghorbel MT, Caputo M, Biglino G, Carrabba M. Biological scaffolds for congenital heart disease. Bioengineering (Basel) 2023; 10(1):57. doi: 10.3390/bioengineering10010057 [Crossref] [ Google Scholar]
- Shinoka T. Development of a tissue-engineering vascular graft for use in congenital heart surgery. EBioMedicine 2014; 1(1):12-3. doi: 10.1016/j.ebiom.2014.10.001 [Crossref] [ Google Scholar]
- Lutter G, Puehler T, Cyganek L, Seiler J, Rogler A, Herberth T. Biodegradable poly-ε-caprolactone scaffolds with ECFCs and iMSCs for tissue-engineered heart valves. Int J Mol Sci 2022; 23(1):527. doi: 10.3390/ijms23010527 [Crossref] [ Google Scholar]
- Roacho-Pérez JA, Garza-Treviño EN, Moncada-Saucedo NK, Carriquiry-Chequer PA, Valencia-Gómez LE, Matthews ER. Artificial scaffolds in cardiac tissue engineering. Life (Basel) 2022; 12(8):1117. doi: 10.3390/life12081117 [Crossref] [ Google Scholar]
- Salih T, Caputo M, Ghorbel MT. Recent advances in hydrogel-based 3D bioprinting and its potential application in the treatment of congenital heart disease. Biomolecules 2024; 14(7):861. doi: 10.3390/biom14070861 [Crossref] [ Google Scholar]
- Vernon MJ, Mela P, Dilley RJ, Jansen S, Doyle BJ, Ihdayhid AR. 3D printing of heart valves. Trends Biotechnol 2024; 42(5):612-30. doi: 10.1016/j.tibtech.2023.11.001 [Crossref] [ Google Scholar]
- Barbulescu GI, Bojin FM, Ordodi VL, Goje ID, Barbulescu AS, Paunescu V. Decellularized extracellular matrix scaffolds for cardiovascular tissue engineering: current techniques and challenges. Int J Mol Sci 2022; 23(21):13040. doi: 10.3390/ijms232113040 [Crossref] [ Google Scholar]
- Lin CH, Hsia K, Ma H, Lee H, Lu JH. In vivo performance of decellularized vascular grafts: a review article. Int J Mol Sci 2018; 19(7):2101. doi: 10.3390/ijms19072101 [Crossref] [ Google Scholar]
- Watanabe S, Sakurai T, Nakamura S, Miyoshi K, Sato M. The combinational use of CRISPR/Cas9 and targeted toxin technology enables efficient isolation of bi-allelic knockout non-human mammalian clones. Int J Mol Sci 2018; 19(4):1075. doi: 10.3390/ijms19041075 [Crossref] [ Google Scholar]
- Wang G, McCain ML, Yang L, He A, Pasqualini FS, Agarwal A. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med 2014; 20(6):616-23. doi: 10.1038/nm.3545 [Crossref] [ Google Scholar]
- Xie C, Zhang YP, Song L, Luo J, Qi W, Hu J. Genome editing with CRISPR/Cas9 in postnatal mice corrects PRKAG2 cardiac syndrome. Cell Res 2016; 26(10):1099-111. doi: 10.1038/cr.2016.101 [Crossref] [ Google Scholar]
- Long C, McAnally JR, Shelton JM, Mireault AA, Bassel-Duby R, Olson EN. Prevention of muscular dystrophy in mice by CRISPR/Cas9-mediated editing of germline DNA. Science 2014; 345(6201):1184-8. doi: 10.1126/science.1254445 [Crossref] [ Google Scholar]
- Stainier DY, Raz E, Lawson ND, Ekker SC, Burdine RD, Eisen JS. Guidelines for morpholino use in zebrafish. PLoS Genet 2017; 13(10):e1007000. doi: 10.1371/journal.pgen.1007000 [Crossref] [ Google Scholar]
- Li S, Liu S, Chen W, Yuan Y, Gu R, Song Y. A novel ZIC3 gene mutation identified in patients with heterotaxy and congenital heart disease. Sci Rep 2018; 8(1):12386. doi: 10.1038/s41598-018-30204-3 [Crossref] [ Google Scholar]
- Paulussen AD, Steyls A, Vanoevelen J, van Tienen FH, Krapels IP, Claes GR. Rare novel variants in the ZIC3 gene cause X-linked heterotaxy. Eur J Hum Genet 2016; 24(12):1783-91. doi: 10.1038/ejhg.2016.91 [Crossref] [ Google Scholar]
- Athota JP, Bhat M, Nampoothiri S, Gowrishankar K, Narayanachar SG, Puttamallesh V. Molecular and clinical studies in 107 Noonan syndrome affected individuals with PTPN11 mutations. BMC Med Genet 2020; 21(1):50. doi: 10.1186/s12881-020-0986-5 [Crossref] [ Google Scholar]
- Wang Y, Li X, Li R, Yang Y, Du J. Identification of novel causal FBN1 mutations in pedigrees of Marfan syndrome. Int J Genomics 2018; 2018:1246516. doi: 10.1155/2018/1246516 [Crossref] [ Google Scholar]
- Lin J, Vora M, Kane NS, Gleason RJ, Padgett RW. Human Marfan and Marfan-like syndrome associated mutations lead to altered trafficking of the type II TGFβ receptor in Caenorhabditis elegans. PLoS One 2019; 14(5):e0216628. doi: 10.1371/journal.pone.0216628 [Crossref] [ Google Scholar]
- Garg V, Kathiriya IS, Barnes R, Schluterman MK, King IN, Butler CA. GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature 2003; 424(6947):443-7. doi: 10.1038/nature01827 [Crossref] [ Google Scholar]
- Gifford CA, Ranade SS, Samarakoon R, Salunga HT, de Soysa TY, Huang Y. Oligogenic inheritance of a human heart disease involving a genetic modifier. Science 2019; 364(6443):865-70. doi: 10.1126/science.aat5056 [Crossref] [ Google Scholar]
- Seok H, Deng R, Cowan DB, Wang DZ. Application of CRISPR-Cas9 gene editing for congenital heart disease. Clin Exp Pediatr 2021; 64(6):269-79. doi: 10.3345/cep.2020.02096 [Crossref] [ Google Scholar]
- Tompkins BA, Balkan W, Winkler J, Gyöngyösi M, Goliasch G, Fernández-Avilés F. Preclinical studies of stem cell therapy for heart disease. Circ Res 2018; 122(7):1006-20. doi: 10.1161/circresaha.117.312486 [Crossref] [ Google Scholar]
- Wehman B, Sharma S, Pietris N, Mishra R, Siddiqui OT, Bigham G. Mesenchymal stem cells preserve neonatal right ventricular function in a porcine model of pressure overload. Am J Physiol Heart Circ Physiol 2016; 310(11):H1816-26. doi: 10.1152/ajpheart.00955.2015 [Crossref] [ Google Scholar]
- Schmuck EG, Hacker TA, Schreier DA, Chesler NC, Wang Z. Beneficial effects of mesenchymal stem cell delivery via a novel cardiac bioscaffold on right ventricles of pulmonary arterial hypertensive rats. Am J Physiol Heart Circ Physiol 2019; 316(5):H1005-13. doi: 10.1152/ajpheart.00091.2018 [Crossref] [ Google Scholar]
- Trac D, Maxwell JT, Brown ME, Xu C, Davis ME. Aggregation of child cardiac progenitor cells into spheres activates notch signaling and improves treatment of right ventricular heart failure. Circ Res 2019; 124(4):526-38. doi: 10.1161/circresaha.118.313845 [Crossref] [ Google Scholar]
- Albertario A, Swim MM, Ahmed EM, Iacobazzi D, Yeong M, Madeddu P. Successful reconstruction of the right ventricular outflow tract by implantation of thymus stem cell engineered graft in growing swine. JACC Basic Transl Sci 2019; 4(3):364-84. doi: 10.1016/j.jacbts.2019.02.001 [Crossref] [ Google Scholar]
- Chery J, Huang S, Gong L, Wang S, Yuan Z, Wong J. Human neonatal thymus mesenchymal stem/stromal cells and chronic right ventricle pressure overload. Bioengineering (Basel) 2019; 6(1):15. doi: 10.3390/bioengineering6010015 [Crossref] [ Google Scholar]
- Nana-Leventaki E, Nana M, Poulianitis N, Sampaziotis D, Perrea D, Sanoudou D. Cardiosphere-derived cells attenuate inflammation, preserve systolic function, and prevent adverse remodeling in rat hearts with experimental autoimmune myocarditis. J Cardiovasc Pharmacol Ther 2019; 24(1):70-7. doi: 10.1177/1074248418784287 [Crossref] [ Google Scholar]
- Tarui S, Ishigami S, Ousaka D, Kasahara S, Ohtsuki S, Sano S, et al. Transcoronary infusion of cardiac progenitor cells in hypoplastic left heart syndrome: three-year follow-up of the transcoronary infusion of cardiac progenitor cells in patients with single-ventricle physiology (TICAP) trial. J Thorac Cardiovasc Surg 2015;150(5):1198-208.e2. 10.1016/j.jtcvs.2015.06.076.
- Brizard CP, Elwood NJ, Kowalski R, Horton SB, Jones BO, Hutchinson D. Safety and feasibility of adjunct autologous cord blood stem cell therapy during the Norwood heart operation. J Thorac Cardiovasc Surg 2023; 166(6):1746-55. doi: 10.1016/j.jtcvs.2023.07.035 [Crossref] [ Google Scholar]
- Burkhart HM, Qureshi MY, Rossano JW, Cantero Peral S, O’Leary PW, Hathcock M. Autologous stem cell therapy for hypoplastic left heart syndrome: safety and feasibility of intraoperative intramyocardial injections. J Thorac Cardiovasc Surg 2019; 158(6):1614-23. doi: 10.1016/j.jtcvs.2019.06.001 [Crossref] [ Google Scholar]
- Ishigami S, Ohtsuki S, Eitoku T, Ousaka D, Kondo M, Kurita Y. Intracoronary cardiac progenitor cells in single ventricle physiology: the PERSEUS (cardiac progenitor cell infusion to treat univentricular heart disease) randomized phase 2 trial. Circ Res 2017; 120(7):1162-73. doi: 10.1161/circresaha.116.310253 [Crossref] [ Google Scholar]
- Noor N, Shapira A, Edri R, Gal I, Wertheim L, Dvir T. 3D printing of personalized thick and perfusable cardiac patches and hearts. Adv Sci (Weinh) 2019; 6(11):1900344. doi: 10.1002/advs.201900344 [Crossref] [ Google Scholar]
- Wang JY, Doudna JA. CRISPR technology: a decade of genome editing is only the beginning. Science 2023; 379(6629):eadd8643. doi: 10.1126/science.add8643 [Crossref] [ Google Scholar]
- Fear VS, Forbes CA, Shaw NC, Farley KO, Mantegna JL, Htun JP. Gene editing and cardiac disease modelling for the interpretation of genetic variants of uncertain significance in congenital heart disease. Stem Cell Res Ther 2023; 14(1):345. doi: 10.1186/s13287-023-03592-1 [Crossref] [ Google Scholar]
- Miyamoto M, Kannan S, Uosaki H, Kakani T, Murphy S, Andersen P, et al. Cardiac progenitors auto-regulate second heart field cell fate via Wnt secretion. bioRxiv [Preprint]. January 31, 2021. Available from: https://www.biorxiv.org/content/10.1101/2021.01.31.428968v1.
- Sidal H, Colakoglu Erkan P, Uslu M, Kocabas F. Development of small-molecule-induced fibroblast expansion technologies. J Tissue Eng Regen Med 2020; 14(10):1476-87. doi: 10.1002/term.3112 [Crossref] [ Google Scholar]
- Zhang H, Pei L, Ouyang Z, Wang H, Chen X, Jiang K. AP-1 activation mediates post-natal cardiomyocyte maturation. Cardiovasc Res 2023; 119(2):536-50. doi: 10.1093/cvr/cvac088 [Crossref] [ Google Scholar]
- Hyun I. The bioethics of stem cell research and therapy. J Clin Invest 2010; 120(1):71-5. doi: 10.1172/jci40435 [Crossref] [ Google Scholar]
- Taylor PL. Research sharing, ethics and public benefit. Nat Biotechnol 2007; 25(4):398-401. doi: 10.1038/nbt0407-398 [Crossref] [ Google Scholar]
- Cuende N, Rasko JE, Koh MB, Dominici M, Ikonomou L. Cell, tissue and gene products with marketing authorization in 2018 worldwide. Cytotherapy 2018; 20(11):1401-13. doi: 10.1016/j.jcyt.2018.09.010 [Crossref] [ Google Scholar]
- Aiyegbusi OL, Macpherson K, Elston L, Myles S, Washington J, Sungum N. Patient and public perspectives on cell and gene therapies: a systematic review. Nat Commun 2020; 11(1):6265. doi: 10.1038/s41467-020-20096-1 [Crossref] [ Google Scholar]
- Alderson P, Bellsham-Revell H, Brierley J, Dedieu N, Heath J, Johnson M. Children’s informed signified and voluntary consent to heart surgery: professionals’ practical perspectives. Nurs Ethics 2022; 29(4):1078-90. doi: 10.1177/09697330211057202 [Crossref] [ Google Scholar]
- Yuan T, Liu Q, Kang J, Gao H, Gui S. High-dose neural stem/progenitor cell transplantation increases engraftment and neuronal distribution and promotes functional recovery in rats after acutely severe spinal cord injury. Stem Cells Int 2019; 2019:9807978. doi: 10.1155/2019/9807978 [Crossref] [ Google Scholar]
- Kawaguchi N, Nakanishi T. Animal disease models and patient-iPS-cell-derived in vitro disease models for cardiovascular biology-how close to disease?. Biology (Basel) 2023; 12(3):468. doi: 10.3390/biology12030468 [Crossref] [ Google Scholar]