Biomed. adv. 2(1):25-33.
doi: 10.34172/bma.11
Original Article
The functional recovery of ischemic human pluripotent stem cell-derived cardiomyocytes by secretome of heat shock-preconditioned umbilical cord mesenchymal stem cells
Fariba Moslem Formal analysis, Methodology, 1, # 
Mojtaba Shafaghi Writing – original draft, 1, # 
Parisa Karamikhaghan Writing – original draft, 1
Mahya Hosseini Formal analysis, Methodology, 1, 2
Hoda Madani Conceptualization, 1
Zahra Haj Amini Conceptualization, 3
Fatemeh Etezadi Writing – review & editing, 1, * 
Sara Pahlavan Writing – review & editing, 1, * 
Author information:
1Department of Stem Cells and Developmental Biology, Cell Science Research Center, Royan Institute for Stem Cell Biology and Technology, ACECR, Tehran, Iran
2Department of Biomedical Sciences, Section Molecular Cell Biology, University Medical Center Groingen, University of Groingen, Groingen, The Netherlands
3School of Nursing and Midwifery, Damavand Branch, Islamic Azad University, Damavand, Iran
#These authors contributed equally to this work.
Abstract
Summary
Introduction:
Cardiovascular diseases are among most prevalent health problems all over the world as well as Iran. Particularly, myocardial infarction comprises 30% of death rate in the world, while 40% in Iran. Moreover, ischemic heart conditions can progress to heart failure. Chronic heart failure may result in serious conditions, making heart transplantation the only remaining clinical solution. However, there is limited number of organ donors which complicates this therapeutic option. Last two decades, cell therapy was introduced as a promising approach to tackle these situations. Mesenchymal stem cells (MSCs) have been widely used in regenerative medicine due to their multipotency and paracrine effects. In this study, we aimed to compare the cardiac repair effects of MSCs’ secretome derived from three sources of adipose, bone marrow and umbilical cord in an in vitro model of hypoxia induced in human induced pluripotent stem cells (hiPSC)-derived cardiomyocytes.
Methods:
Hypoxia was induced in hiPSC-derived cardiomyocytes and neonatal mouse cardiomyocytes using 1 mM H2O2 for 4 hours. Hypoxia induction was confirmed by translocation of Hif-1α into nucleus as visualized by immunostaining of cells pre and post-hypoxia. The cardiomyocytes were then subjected to indirect co-culture with heat shock-preconditioned MSCs post hypoxia, in order to receive MSCs secretome.
Findings:
Electrophysiological evaluation of cardiomyocytes as well as their contraction analysis post-MSCs secretome reperfusion, showed that umbilical cord MSCs could superiorly recover excitation-contraction coupling in cardiomyocytes.
Conclusion:
In conclusion, umbilical cord MSCs might be the best MSCs source for cardiac cell therapy or its secretome as a cell free approach.
Keywords: Myocardial infarction, Cell therapy, Umbilical cord, Mesenchymal stem cell
Copyright and License Information
© 2025 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
This work was supported by a grant from Royan Institute and Iran National Science Foundation (grant number: 95849387).
Introduction
To date, most of the cardiac cell therapy studies have been done using bone marrow-derived cells, because bone marrow is a rich source of all kinds of stem cells and precursors. Furthermore, it is possible to separate bone marrow cells with easy methods such as centrifugation. But the results obtained from these studies were contradictory. In some clinical trials, such as BOOST and REPAIR-AMI, it has been shown that bone marrow stem cells can improve the function of the left ventricle,1 while in recent trials they did not observe a significant improvement in the function of the heart.2 It is north worthy though that the injection methods might influence the efficacy of cell therapy. For example, in a study in which bone marrow cells were injected to patients hearts intracoronary, only 3% of the injected cells remained within myocardium after 1 hour.3 Compared to the intracoronary method, in another method called transendocardial, the injected cells had a longer residence and as a result, they showed better efficiency.4 Moreover, the different results obtained from different studies can be attributed to the heterogeneity of the cells in the bone marrow. So that the higher the amount of cells with CD34 and CD31 surface antigens in the injected cell population, the greater the improvement in the heart function.
Mesenchymal stem cells (MSCs) are multipotent cells and have the ability to differentiate into adipogenic, osteogenic and chondrogenic lineages.5 The main source of these cells is in the bone marrow, however, they are also found in some tissues such as umbilical cord, fat, muscle and endometrium. MSCs can be used as a product and in an allogeneic form due to the lack of expression of some antigens such as MHC class 2, which play an essential role in transplant rejection. These cells can naturally reside in all post-embryonic organs and tissues,6 but they are often found in bone marrow and constitute 0.001% to 0.01% of bone marrow cells. Isolated MSCs are a heterogeneous cell population, which are characterized based on the properties including adhesion to a plate, formation of fibroblastic colonies and differentiation into three cell types of bone, cartilage and fat. They express surface markers CD73, CD90 and CD105 and are negative for CD11b, CD14, CD34, CD45 and HLA-DR markers.5
Cell-based therapies offer new hopes for regenerating the damaged heart. Among various cell sources, the use of MSCs have shown promising results. The first clinical trial for myocardial infarction was conducted between 2002 and 2005 using unfractionated high heterogeneous adult bone marrow-derived mononuclear cells (BM-MNCs) and initial positive results showed the safety of these cells. However, contradictory results were obtained in several first-generation clinical trials, which might be attributed to differences in the design of the experiment, the separation of cells and the analysis of the results. And this made the announcement of final results difficult. In general, the results of these studies proposed a reparative role for MSCs by various mechanisms. A study of infarcted hearts of female pigs receiving allogeneic bone marrow-derived mesenchymal stromal cell (BM-MSCs) showed that these cells could be differentiated into heart cells.7 But in another study of the mouse myocardial infarction model, transplanted human MSCs were not visible a few weeks after injection.8 Also, among the MSCs injected into the healthy pig heart, only 2% remained after two weeks and there was no evidence of differentiation of MSCs into heart cells. Therefore, the differentiation of MSCs into cardiomyocyte seems not to be the underlying mechanism. Another possible mechanism is the fusion of MSCs with heart cells in the same area. Although this phenomenon is rarely observed, it can be one of the reasons for the positive effect of MSCs on cardiac cell regeneration. Two other possible mechanisms for the effectiveness of MSCs include recruitment of endogenous cardiac stem cells9 and paracrine effects of MSCs.10 There is currently disagreement on whether MSCs need to be transplanted directly to the affected site or can apply their effects by systemic injection. Cell transplantation can increase the potential of cell-cell communication and also increase the release of immune and trophic regulating factors in the body. However, the ischemia microenvironment can create a toxic area for MSCs and cause serious disruptions in the survival of these cells. Experiments on small animals have shown that MSCs cannot remain and/or survive in the transplant site. And if these cells do not settle in the host tissue, most of them will die within a month. The lack of MSCs in the ischemia microenvironment can be exacerbated in the presence of ROS because it prevents cellular adhesion to the extracellular matrix.11 Therefore, the probability of cardiac repair due to paracrine factors is increased which might include growth factors, cytokines and other effective signaling molecules in both forms of secretory factors and extracellular vesicles. These factors can provide a suitable microenvironment to support reparative events, stimulate angiogenesis and prevent wider tissue damage. Takahashi et al showed that cytokines from MSCs maintain the contractile properties of myocardium, prevent apoptosis of heart cells, and stimulate the formation of new blood vessels in the affected area.12 In some studies, researchers tried to improve the reparative effects of MSCs by preconditioning strategies such as oxidative stress, heat shock or genetic engineering of MSCs before transplantation.13,14,15 Mangi et al increased AKT1 expression in MSCs which resulted in a more efficient myocardial repair.16
The use of in vitro models based on isolated heart cells can be an alternative to animal models. Although these models cannot create the complexity of the heart for us, they have the advantage of showing the direct effect of the specific treatment on the heart cells without considering other intervening factors. Also in ischemic models in vitro, intercellular signaling pathways that have been altered due to ischemia can be examined and appropriate interventions can be found to improve it. In this study, we intend to compare the effect of MSCs secretome from different sources of bone marrow, fat and umbilical cord on cardiac repair by using human induced pluripotent stem cells-derived cardiomyocytes (hiPSC-CM) and neonatal mouse cardiomyocytes (NMCM). The hypoxia was induced by H2O2 treatment of cardiomyocytes and was confirmed using Hif-1α staining. The hypoxic cardiomyocytes were indirectly co-cultured with heat shock-preconditioned MSCs from various sources to receive fresh secretome. The secretome of heat shock-preconditioned umbilical cord MSCs (UC-MSCs) resulted in the best reparative effect on beating frequency and cardiomyocytes excitability as well as contraction duration. The MSCs secretome changed the expression of PI3K, mTOR, Caspase3, and HIF-1α in hiPSC-CM which might be the intracellular effector molecules for reparative effect.
Methods
Human induced pluripotent stem cell (hiPSC) expansion and differentiation
Bombay human induced pluripotent stem cell (Bam-iPSC) line was received from Royan Institute cell bank.17 The expansion of iPSCs in static suspension culture and subsequent differentiation, was performed as previously described.18 Briefly, the iPSCs were expanded in ultra-low attachment plates in a medium composed of DMEM/F12 (Gibco) supplemented with 20% knock-out serum replacement (Gibco), 100 ngmL-1 bFGF (Sigma), 1% non-essential amino acids (NEAA, Gibco), 1% penicillin and streptomycin (Pen/Strep, Gibco), 1% insulin-transferrin-selenite (Gibco), 1% GlutaMAX (Gibco), and 0.1 mm β-mercaptoethanol. Differentiation was induced on 5-day-old spheroids with an average diameter of 175 ± 25 μm. The expansion medium was replaced by differentiation medium composed of RPMI1640 (Gibco) supplemented with 2% B27 minus vitamin A (Gibco), 1% NEAA, 1% Pen/Strep, 1% GlutaMAX (Gibco), 0.1 mM β-mercaptoethanol, and 12 μM of small molecule (SM) CHIR99021 (CHIR;041-0004, Stemgent) for induction of Wnt/β-catenin signaling pathway. After 24 hours incubation, spheroids were washed with PBS and maintained in the fresh SM-free differentiation medium for 1 day. With the onset of day 3, spheroids were incubated with fresh differentiation medium containing 5 μm IWP2 (3533, Tocris Bioscience) for Wnt signaling inhibition, 5 μM SB431542 (S4317, Sigma–Aldrich) for inhibition of the activin receptor-like kinase receptors, and 5 μm purmorphamine (Pur; 04–0009, Stemgent) for sonic hedgehog pathway induction. After 48 hours of 3-SM incubation, the spheroids were washed with phosphate-buffered saline (PBS) and incubated in new SM-free differentiation medium. The media was exchanged every other day until day 15.
Isolation of neonatal mouse cardiomyocytes
NMCMs were isolated from one-day-old pups according to a protocol described previously.19 All animal studies were performed in accordance with guidelines approved by the Ethics Committee of Royan Institute (Tehran, Iran IR.ACECR.ROYAN.REC.1398.192). Briefly, the hearts were excised and rapidly rinsed in cold Hank’s balanced salt solution (HBSS, Gibco, USA). All non-cardiac tissues and the atria were carefully dissociated from the ventricles. Then, the ventricles were further rinsed in cold HBSS to remove any remaining blood and then they were minced into small pieces. Next, the pieces were treated with HBSS/trypsin (0.5 mg/mL) overnight at 4 °C on an orbital shaker at 80 rpm. After the pre-digestion step, warm culture medium that consisted of 75% Dulbecco’s modified eagle medium (DMEM, Gibco), 25% Media 199 (M-199, Gibco), 1% pen/strep (Gibco), 1% L-glutamine (Gibco) and 1% 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid (HEPES, Gibco) was added to the trypsinized heart tissues. After discarding the pre-digestion media, the heart tissue was subjected to serial digestions with an HBSS/collagenase II (0.8 mg/mL) solution on an orbital shaker at 37 °C. Following the digestion steps, we collected the supernatants, centrifuged them at 800 rpm for 5 minutes at room temperature (RT), and resuspended the cell pellet in culture media composed of DMEM, M-199, 1% pen/strep, 1% L-glutamine, 1% HEPES, 20% horse serum (Gibco), and 10% fetal bovine serum (FBS, Invitrogen, Carlsbad, CA, USA). The cell suspension was preplated on 0.1% gelatin-coated T-75 flasks for 1 hour at 37 °C and 5% CO2 to remove the isolated cardiac fibroblasts and obtain a homogeneous population of cardiomyocytes. Finally, the preplate supernatant was collected and centrifuged, and the pellet of NMCMs was resuspended in culture media.20
Cultivation of mesenchymal stem cells from three sources of adipose tissue, umbilical cord and bone marrow
Adipose-derived mesenchymal stem cells (AD-MSCs) and BM-MSCs were received from the Royan Institute cell bank, while umbilical cord mesenchymal stem cells (UC-MSCs) were purchased from Royan Stem Cell Technology Co & Cord Blood Bank (Supplementary file 1, Figure S1). The culture medium of these cells was composed of DMEM-High Glucose, FBS 15%, NEAA1% and Pen/Strep1%. After reaching a confluency of 70%, they were subcultured. For heat shock pre-treatment, MSCs were incubated at 41 °C for 45 minutes.
Hypoxia induction
In order to find the effective concentration of hydrogen peroxide and the appropriate incubation time for hypoxia induction, 13 × 103 Neonatal Rat Cardiomyocyte (NRCM) that were cultured in each well of a 96 well plate was subjected to serial concentrations of H2O2 (0.5, 0.75, 1, 1.25, 1.5, 1.75, 2, 3, 5 and 10 mM) for 4, 12 and 24 hours. The H2O2-induced oxidative stress was evaluated by lactate dehydrogenase (LDH) release and Hif-1α nuclear translocation using the appropriate assays. The hypoxia was induced in hiPSC-CM using the effective H2O2 concentration and appropriate duration which was obtained from the NRCM test.
Lactate dehydrogenase assay
To perform LDH assay (CytoTox-ONETM Homogeneous Membrane Integrity Assay), the CytoTox-ONETM Reagent and Equilibration Buffer was thawed at RT and mixed. Then, 100 μl of this mixture was added to the same volume of media collected from each well of a 96 well plate containing treated cells with serial concentrations of H2O2. The final volume (200 μl) was then transferred to a dark plate and the absorbance was measured using a microplate reader (Multiskan Spectrum, Thermo Scientific) at 580 nm.
Immunofluorescence staining
NRCM or hiPSC-CM were washed with PBS, fixed with 4% (w/v) paraformaldehyde at RT for 15 minutes, washed three times with washing buffer (PBS/0.1% Tween 20), permeabilized with 0.2% Triton X-100 in PBS for 15 minutes, and blocked with 1% (v/v) bovine serum for 1 hour. Primary antibodies diluted in blocking buffer (PBS/0.2%Triton X-100/1% bovine serum) (1:100) were added to the cells and incubated overnight at 4 °C. After incubation, the cells were washed three times (5 minutes each) with washing buffer. Secondary antibodies diluted in blocking buffer (1:500) were added to the cells and incubated for 1 hour at RT. The cells were subsequently washed three times with washing buffer. The nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; D-8417, Sigma-Aldrich) and photographed using an Olympus IX71 microscope equipped with a DP72 digital camera. The primary and secondary antibodies are listed in Table S1.
Flow Cytometry
hiPSC and hiPSC-CM were dissociated into single cells using trypsin‐EDTA (25300‐062; Gibco) and fixed in 4% paraformaldehyde for 20 minutes at 4 °C. Samples were permeabilized in 0.5% Triton X‐100 for 20 minutes at RT and blocked with 10% heat‐inactivated bovine serum albumin (BSA) in PBS for 45 minutes at RT. Cells were incubated with primary antibody overnight at 4 °C, then washed with washing buffer two times. The secondary antibody was applied for 45 minutes at 4 °C, followed by two times washing steps with washing buffer. Cells were then analyzed using a flow cytometer (FACSCalibur; BD Biosciences, San Jose, CA) and flowing software, version 2.5.1 (BD Biosciences). Permeabilization step was skipped for surface markers.
Indirect co-culture of hiPSC and MSCs
In order to supply fresh MSCs secretome, heat shock pre-conditioned MSCs that were cultured on transwell cell culture inserts (Corning® Transwell® 24 well plates), were transferred to each well of a 24 well plate containing H2O2-treated hiPSC-CM for 48 hours. The hiPSC-CM were then subjected to functional and molecular assays.
Field potential recording
Extracellular field potential was recorded using microelectrode array (MEA) data acquisition system (Multi Channel Systems in Reutlingen, Germany). The MEA plates had a grid of 60 titanium nitride electrodes (30 μm) with a 200 μm inter-electrode distance. Before the experiment, the MEA plates were sterilized, hydrophilized with FBS for 30 minutes, rinsed with sterile water, and coated with matrigel. Then, the hiPSC-CM spheroids were placed in the center of a sterilized MEA plate. Subsequently, the MEAs were connected to a head-stage amplifier. The extracellular field potentials were recorded at a sampling rate of 10 kHz, and all the measurements were conducted at 37 °C. Recordings lasted for 60 seconds and the resulting signals were analyzed for filed potential duration (FPD) and interspike interval (ISI) using Cardio2D + software.
Ca2+ imaging
Ca2+ transient measurements were performed on Olympus IX71 microscope equipped with a DP72 digital camera, after loading the hiPSC-CM spheroids with 1mM of Fura-2 AM. The recordings were obtained from spontaneous Ca2+ release that stimulated by spontaneous action potentials in hiPSC-CM. Cells were imaged pre- and post-hypoxia as well as post-MSCs secretome treatment. The Ca2+ transient duration was compared at the abovementioned treatments.
Real-time quantitative reverse transcription PCR
RNA isolation was carried out using RNeasy micro kit (QIAGEN, 74004, USA) according to the manufacturer’s protocol. The resultant RNA was reverse-transcribed into cDNA using Easy cDNA Synthesis Kit (A101161, Pars Tous Biotechnology) and then diluted to 25 ng/μL for real-time quantitative reverse transcription PCR (qRT-PCR) using Applied BiosystemsTM StepOneTM Real-Time PCR System (Fischer scientific, Canada). GAPDH was used as the housekeeping gene. The primers are listed in Table S2.
Statistical analysis
Most experiments were performed in three biological replicates, while a few was done in one replicate. Statistical analysis was performed using the GraphPad Prism software (9.0.2) and all results were presented as mean ± standard error of mean (SEM). Significant differences between groups were calculated using proper statistical tests, including an unpaired t test or one-way analysis of variance (ANOVA). A statistically significant level was considered as P < 0.05.
Results
Successful differentiation of hiPSCs into Cardiomyocytes
Bam-iPSCs were expanded and differentiated to cardiomyocytes according to our previously published protocol21 (Figure 1A) Initially, hiPSCs were cultured and expanded in adherent culture system (Figure 1B, left panel), while the culture system changed to static suspension before starting the differentiation protocol (Figure 1B, middle panel). The Bam-iPSC colonies with 175 ± 25 μm diameter were subjected to small molecules for cardiogenic differentiation, which resulted into spontaneously beating spheroids at day 7 of differentiation induction (Figure 1B, right panel) with 31 ± 5 beats per minute (BMP) (Figure 1C). While more than 90% of Bam-iPSC were OCT4+ (Figure 1D & E), their cardiomyocyte derivative was cTNT+ with abundance of ~80% (Figure 1F & G). Flow cytometry analysis confirmed the immunofluorescence results, where NANOG + and cTNT + cell populations exchanged from 90.35% and 2.70% into 2.14% and 91.75%, respectively (Figure 1H). Furthermore, the expression of cardiomyocyte-specific genes (cTNT, MLC2v and α-MHC) upregulated in Bam iPSC-CM. The excitation-contraction coupling was also assessed in differentiated cardiomyocyte spheroids which showed a field potential duration of 780 ± 25 ms, an interspike interval of 2 ± 0.35 s and contraction duration of 998 ± 250 ms. Altogether, these results indicated the efficient cardiomyocyte differentiation at cellular and functional level.
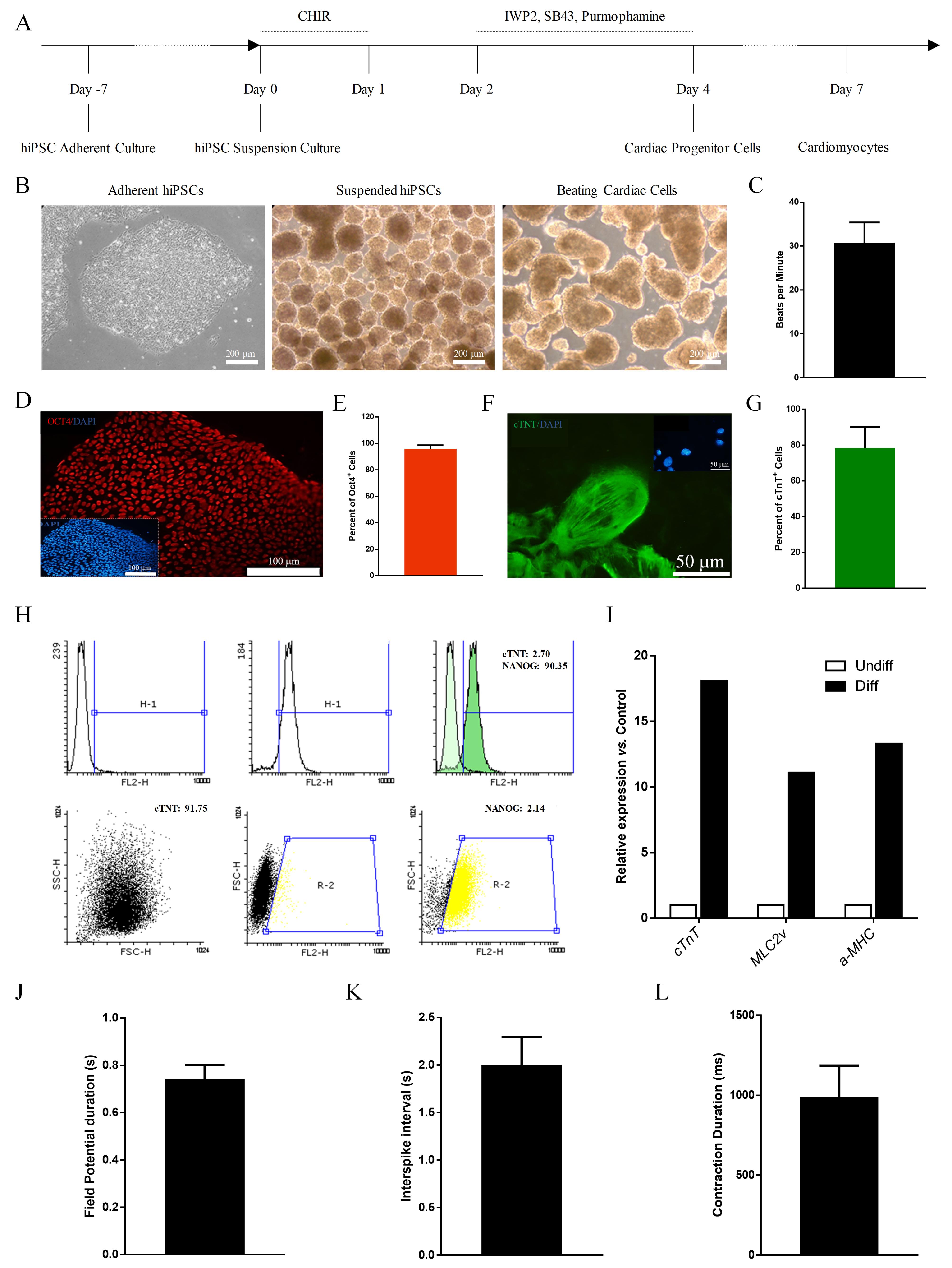
Figure 1.
Bam iPSC differentiation and characterization. (A) Time line of cardiogenic differentiation. (B) Light microscopy images of hiPSC and hiPSC-CM spheroids. C) Spontaneous beating rates of hiPSC-CM. D-E) Immunostaining of hiPSC for OCT4 and quantification of OCT4+ cells. (F-G) Immunostaining of hiPSC-CM for cTNT and quantification of cTNT+ cells. (H) Flow cytometry of hiPSC and hiPSC-CM for NANOG and cTNT. (I) Gene expression analysis of cardiac-specific markers in hiPSC and hiPSC-CM. J-K) Filed potential duration and interspike interval in hiPSC-CM spheroids as measures of excitation. (L) Contraction analysis
.
Bam iPSC differentiation and characterization. (A) Time line of cardiogenic differentiation. (B) Light microscopy images of hiPSC and hiPSC-CM spheroids. C) Spontaneous beating rates of hiPSC-CM. D-E) Immunostaining of hiPSC for OCT4 and quantification of OCT4+ cells. (F-G) Immunostaining of hiPSC-CM for cTNT and quantification of cTNT+ cells. (H) Flow cytometry of hiPSC and hiPSC-CM for NANOG and cTNT. (I) Gene expression analysis of cardiac-specific markers in hiPSC and hiPSC-CM. J-K) Filed potential duration and interspike interval in hiPSC-CM spheroids as measures of excitation. (L) Contraction analysis
H2O2 treatment induced oxidative stress in NRCM
In order to obtain the effective concentration and appropriate incubation time for oxidative stress induction in NRCM, these cardiomyocytes were treated with a serial concentration of H2O2 and evaluated at three incremental time points. As shown in Figure 2A, the concentration of 0.75 mM and above for 4 hours, resulted in a substantial increase in LDH release which is a sign of oxidative stress in NRCM (Figure 2A). When tested by immunostaining, the concentration of 1 mM H2O2 resulted in Hif-1α nuclear translocation as a sign of oxidative stress. Therefore, the effective concentration and the appropriate incubation time were determined as 1 mM and 4 hours, respectively.
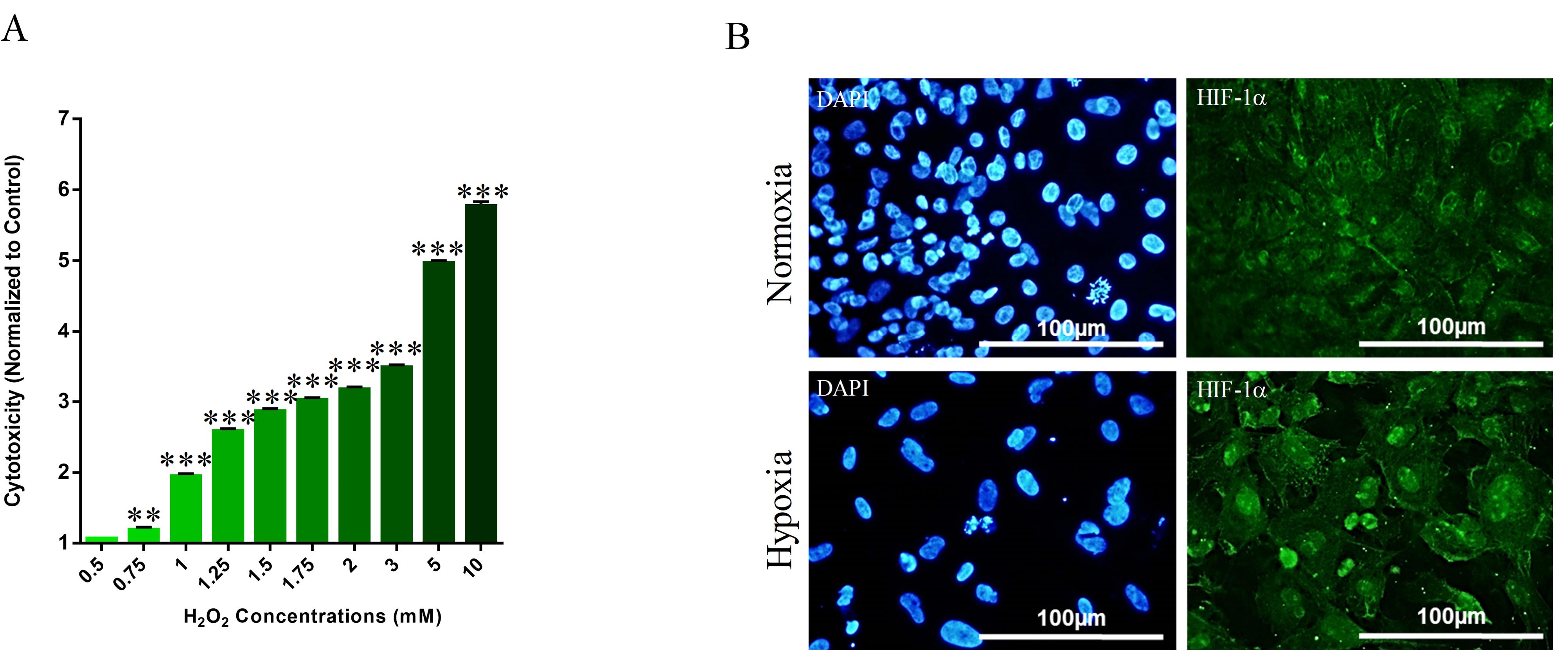
Figure 2.
Hypoxia induction in NMCM. (A) LDH release assay to determine the effective H2O2 concentration. (B) H2O2-induced Hif-1α nuclear translocation
.
Hypoxia induction in NMCM. (A) LDH release assay to determine the effective H2O2 concentration. (B) H2O2-induced Hif-1α nuclear translocation
UC-MSCs performed the best in the recovery of hypoxic cardiomyocytes’ excitation-contraction coupling
Bam iPSC-CM spheroids were assessed with respect to their excitability and contractility before being subjected to oxidative stress by 1 mM H2O2 (Figure 3A-E, Before Hypoxia). Following 4h of H2O2 treatment, the field potentials and Ca2+ transients were recorded and analyzed (Figure 3A-E, after hypoxia). Then, the Bam iPSC-CM spheroids were indirectly co-cultured with heat shock pre-conditioned MSCs from three sources of bone marrow, adipose tissue and umbilical cord for 48 hours using transwell. The field potential recording and Ca2+ transient measurements were performed after cardiomyocytes treatments with secretome of heat shock pre-conditioned MSCs, which was achieved through indirect co-culture (Figure 3A-E, after reperfusion). As shown in Figure 3B and Table 1, the BMP decreased following oxidative stress and partially recovered by AD-MSCs, while fully recovered by BM-MSCs and UC-MSCs. FPD increased following hypoxia and returned to prehypoxia values and even lower after all types of MSCs secretome treatment (Figure 3C). The hypoxia-induced prolongation of ISI was partially rescued by AD-MSCs and BM-MSCs, while fully recovered and even reached to lower value by UC-MSCs, which indicates the reparative effect of MSCs secretome specially UC-MSCs. Although secretome of all types of MSCs had a reparative effect on hypoxic cardiomyocytes’ excitability, the UC-MSCs was the only secretome with recovery effect on Ca2+ transient duration (Figure 3E). It is north worthy to mention that oxidative stress inhibited Ca2+ transient in Bam iPSC-CM spheroids (Figure 3E).
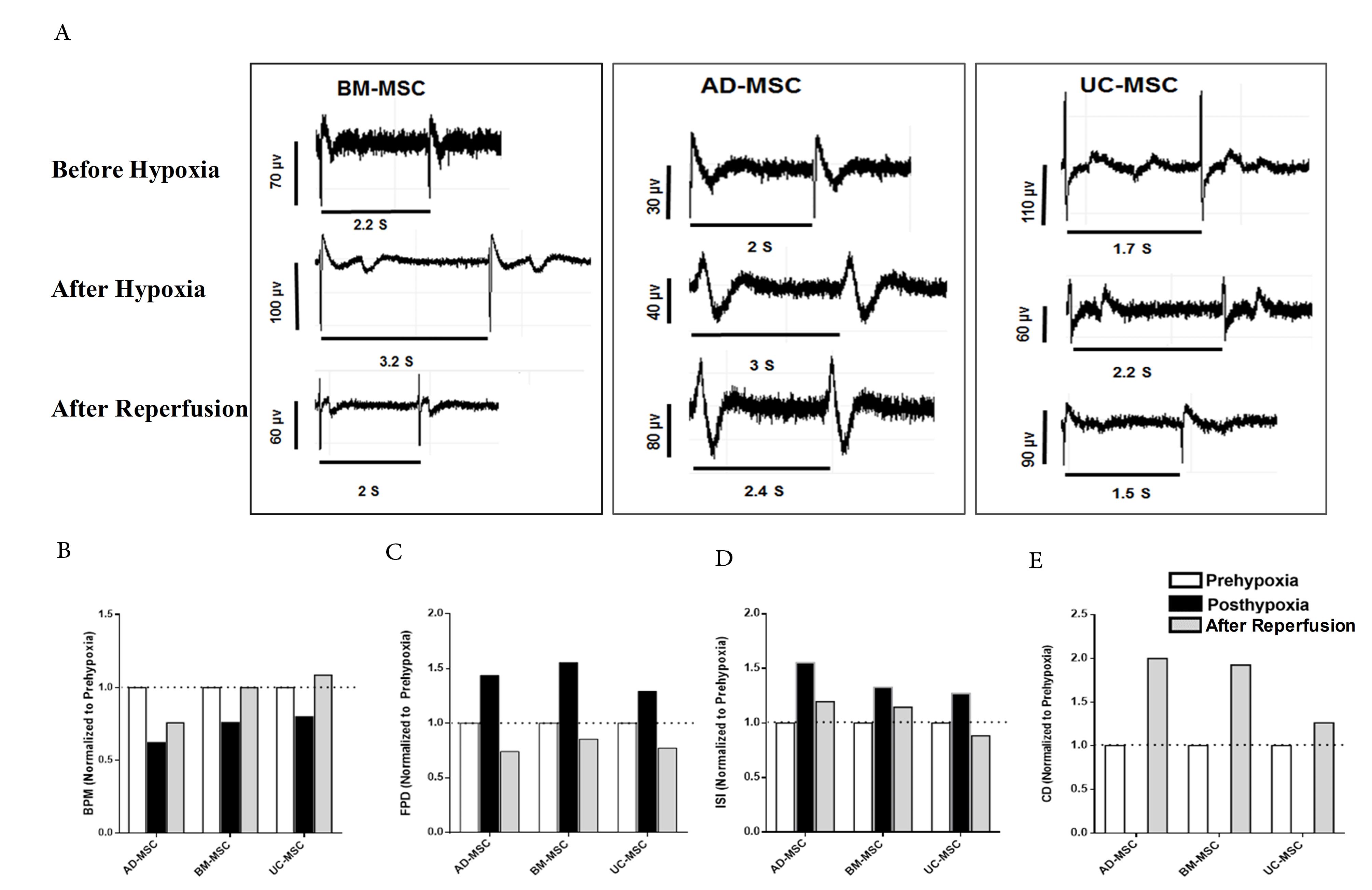
Figure 3.
Functional assessment of hypoxia and reperfused hiPSC-CM. (A) Representative electrograms of hiPSC-CM spheroids before hypoxia, after hypoxia and after reperfusion with MSCs secretome. (B) Spontaneous beating rate, field potential duration (FPD), interspike interval (ISI) and contraction duration (CO) of hiPSC-CM spheroids before hypoxia, after hypoxia and after reperfusion with MSCs secretome
.
Functional assessment of hypoxia and reperfused hiPSC-CM. (A) Representative electrograms of hiPSC-CM spheroids before hypoxia, after hypoxia and after reperfusion with MSCs secretome. (B) Spontaneous beating rate, field potential duration (FPD), interspike interval (ISI) and contraction duration (CO) of hiPSC-CM spheroids before hypoxia, after hypoxia and after reperfusion with MSCs secretome
Table 1.
Electrophysiological properties of hiPSC-CM spheroids before hypoxia, after hypoxia and after reperfusion
|
Group/Treatment
|
Beat/min
|
FPD
|
ISI
|
| Control |
34 |
0.31 |
1.80 |
| BM-MSCs |
Before hypoxia |
25 |
0.5 |
2.55 |
| After hypoxia |
19 |
1.3 |
3.19 |
| After reperfusion |
25 |
0.66 |
2.71 |
| AD-MSCs |
Before hypoxia |
29 |
0.39 |
2.07 |
| After hypoxia |
18 |
1.07 |
3.29 |
| After reperfusion |
22 |
0.51 |
2.51 |
| UC-MSCs |
Before hypoxia |
35 |
0.49 |
1.72 |
| After hypoxia |
28 |
0.64 |
2.19 |
| After reperfusion |
35 |
0.38 |
1.52 |
Abbreviations: BM-MSCs, bone marrow-derived mesenchymal stromal cell; AD-MSCs, adipose-derived mesenchymal stem cells, UCMSCs, umbilical cord mesenchymal stem cell; FPD, filed potential duration; ISI, interspike interval.
MSCs secretome increased the cell survival-related genes expression
In order to study the mechanisms involved in the reparative effect of MSCs secretome, the Bam iPSC-CM were subjected to gene expression analysis after reperfusion. As shown in Figure 4, the expression of PI3K, mTOR and Hif-1α was increased by all types of MSCs secretome. In contrary, BAX and Caspase3 were downregulated following reperfusion (Figure 4). While BM-MSCs and UC-MSCs increased BCL2 expression, AD-MSCs did not regulate it (Figure 4).
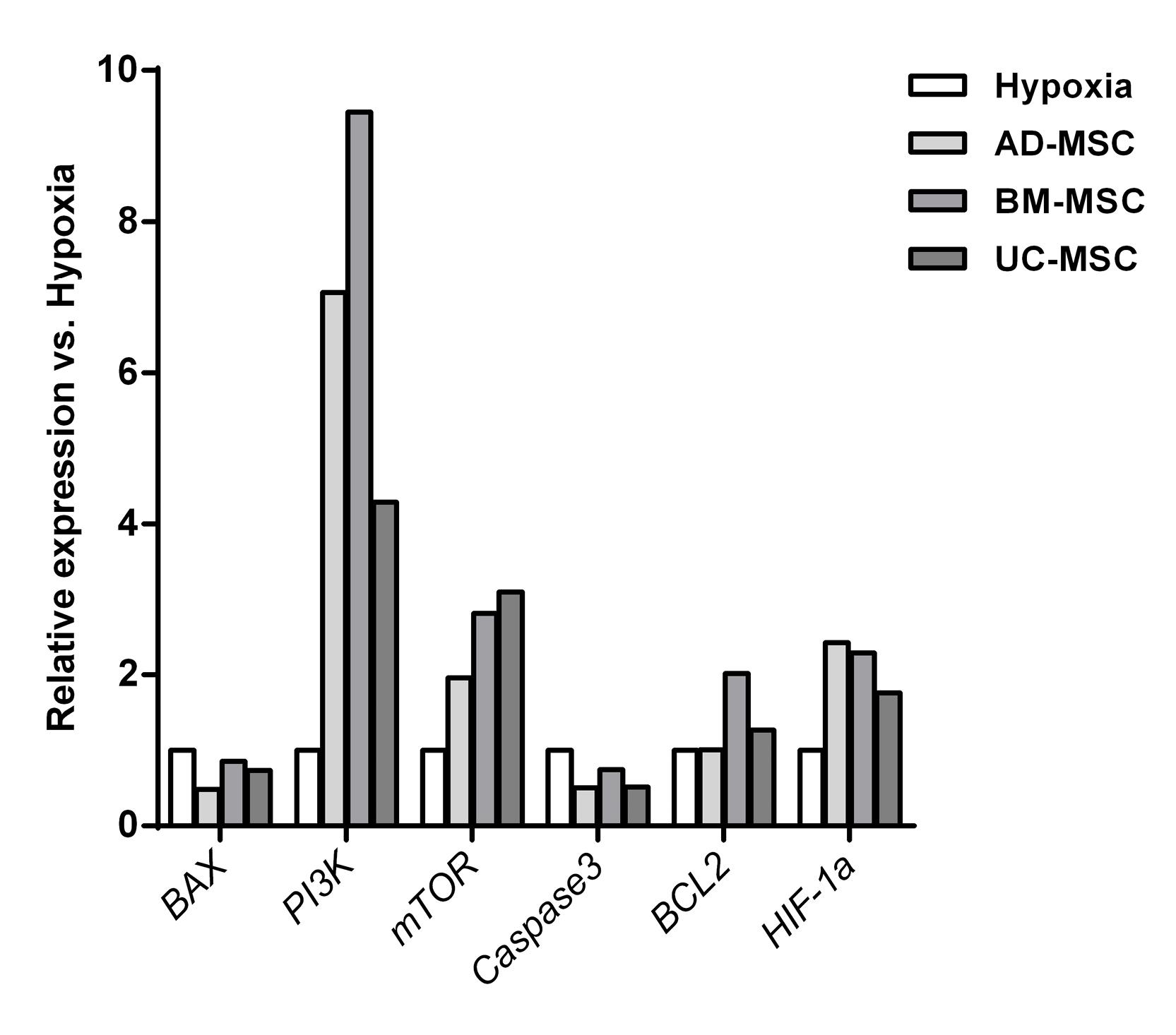
Figure 4.
Gene expression analysis in hiPSC-CM before hypoxia, after hypoxia and after reperfusion with MSCs secretome
.
Gene expression analysis in hiPSC-CM before hypoxia, after hypoxia and after reperfusion with MSCs secretome
Discussion
MSCs have long been the focus of studies for heart repair following an ischemic damage. While there has been an agreement on the reparative effect through paracrine factors, but there is still controversy on the most effective MSCs source for heart repair. In the present study, we investigated the reparative effect of MSCs secretome on hiPSC-derived cardiomyocytes which was subjected to oxidative stress by H2O2 treatment. The hypoxic hiPSC-CM was used as an in vitro model of ischemic damage and its indirect co-culture with three types of MSCs provided the opportunity to model the receipt of fresh MSCs secretome. Furthermore, the heat shock pre-conditioning of MSCs assessed the possibility of more efficient secretome for reparative effect. The evaluation of the functional characteristics of cardiomyocytes after secretome treatment showed that although all three MSCs sources have helped to improve the excitation-contraction coupling, the UC-MSCs performed the best. Messerli’s team also used N2 neuroblastoma line with the aim of evaluating the effect of extracellular vesicles obtained from Wharton’s jelly mesenchyme on neuronal survival and regeneration in an in vitro ischemia-reperfusion model. After establishing the model using minimal oxygen-glucose/reoxygenation environment, Wharton’s jelly mesenchyme extracellular vesicles were used, and its effectiveness on the protection of damaged neurons and the regeneration was observed. This effect occurred through increasing the phosphorylation of BAD from the Bcl-2 family, as well as interfering with the level of Caspase3 gene expression. Thus, it seems that these vesicles supported the cells by controlling the apoptotic pathway.22 Previously, a study was conducted on the effect of this mesenchyme on cell death induced by H2O2 in rat myoblast cells, kidney cells and human liver cells.23 The results were in agreement with the results of Messerli’s team. These extracellular vesicles of Wharton’s jelly MSCs seems to help the recovery of damaged tissue in all types of damaged cells by controlling apoptotic pathways and stimulating cell proliferation.24 The effect of bone marrow mesenchyme exosomes transduced with GATA-4 gene on the cardiomyocyte ischemic model, have been also attributed to the increased expression of p-53.25 The studies by Cantoni et al and Shabbir et al, in 2012 and 2010 also showed that PI3K/Akt, Akt/eNOS/Bcl-2, JAK/STAT, ERK and other MAP kinase pathways are improved by mesenchymal treatments.26,27 These signaling pathways are activated to induce survival, proliferation, anti-apoptosis, extracellular matrix rearrangement, anti-inflammation and angiogenesis.28 The current study also showed that secretome from all three sources of MSCs (Ad-MSCs, BM-MSCs and UC-MSCs) upregulated the genes involved in cell proliferation and survival such as PI3K and downregulated apoptosis genes such as BCL-2.
In 2017, Zakikhan and colleagues investigated the effect of conditioned medium from adipose-derived MSCs on the survival and proliferation of hepatocyte cells.29 This study was performed in a comparative way between the hypoxia pre-conditioned and normoxia pre-conditioned MSCs. The results showed that the hypoxia pre-conditioning induces the release of enriched and more efficient conditioned medium. Although both conditioned media led to better performance of hepatocytes, the conditioned medium obtained from hypoxia pre-conditioned MSCs caused a further increase in glycogen storage and proliferation rate in hepatocyte cells. In the current study, the heat shock preconditioning along with indirect co-culture of MSCs and iPSC-CM was used to improve the reparative effect.
Conclusion
In conclusion, UC-MSCs performed the best among the three tested MSCs with respect to excitation-contraction coupling recovery of hypoxic iPSC-CM. Therefore, the secretome of umbilical cord MSCs as well as their exosomes might be a better candidate for pre-clinical studies and clinical trials of cell-based therapies for ischemic heart diseases such as myocardial infarction.
Competing Interests
The authors declare there are no conflicts of interest.
Ethical Approval
The study was performed in accordance with guidelines approved by the Ethics Committee of Royan Institute (Tehran, Iran IR.ACECR.ROYAN.REC.1398.192).
Supplementary Files
Supplementary file 1 contains Figure S1 and Tables S1-S2.
(pdf)
Acknowledgements
The authors would like to thank Hassan Ansari for his help and support.
References
- Zohlnhöfer D, Ott I, Mehilli J, Schömig K, Michalk F, Ibrahim T. Stem cell mobilization by granulocyte colony-stimulating factor in patients with acute myocardial infarction: a randomized controlled trial. Jama 2006; 295(9):1003-10. doi: 10.1001/jama.295.9.1003 [Crossref] [ Google Scholar]
- Traverse JH, Henry TD, Ellis SG, Pepine CJ, Willerson JT, Zhao DX. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. JAMA 2011; 306(19):2110-9. doi: 10.1001/jama.2011.1670 [Crossref] [ Google Scholar]
- Hertenstein B, Wollert KC, Hofmann M, Meyer GP, Arseniev L, Gasner A. Monitoring of bone marrow cell homing in the infarcted human myocardium by PET. Blood 2004; 104(11):2696. doi: 10.1182/blood.V104.11.2696.2696 [Crossref] [ Google Scholar]
- Vrtovec B, Poglajen G, Lezaic L, Sever M, Socan A, Domanovic D. Comparison of transendocardial and intracoronary CD34 + cell transplantation in patients with nonischemic dilated cardiomyopathy. Circulation 2013; 128(11 Suppl 1):S42-9. doi: 10.1161/circulationaha.112.000230 [Crossref] [ Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D. Minimal criteria for defining multipotent mesenchymal stromal cells The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8(4):315-7. doi: 10.1080/14653240600855905 [Crossref] [ Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008; 3(3):301-13. doi: 10.1016/j.stem.2008.07.003 [Crossref] [ Google Scholar]
- Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res 2010; 107(7):913-22. doi: 10.1161/circresaha.110.222703 [Crossref] [ Google Scholar]
- Li Z, Lee A, Huang M, Chun H, Chung J, Chu P. Imaging survival and function of transplanted cardiac resident stem cells. J Am Coll Cardiol 2009; 53(14):1229-40. doi: 10.1016/j.jacc.2008.12.036 [Crossref] [ Google Scholar]
- Mazhari R, Hare JM. Mechanisms of action of mesenchymal stem cells in cardiac repair: potential influences on the cardiac stem cell niche. Nat Clin Pract Cardiovasc Med 2007; 4 Suppl 1:S21-6. doi: 10.1038/ncpcardio0770 [Crossref] [ Google Scholar]
- Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 2009; 5(1):54-63. doi: 10.1016/j.stem.2009.05.003 [Crossref] [ Google Scholar]
- Song H, Cha MJ, Song BW, Kim IK, Chang W, Lim S. Reactive oxygen species inhibit adhesion of mesenchymal stem cells implanted into ischemic myocardium via interference of focal adhesion complex. Stem Cells 2010; 28(3):555-63. doi: 10.1002/stem.302 [Crossref] [ Google Scholar]
- Takahashi M, Li T-S, Suzuki R, Kobayashi T, Ito H, Ikeda Y. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. American Journal of Physiology-Heart and Circulatory Physiology 2006; 291(2):H886-H93. doi: 10.1152/ajpheart.00142.2006 [Crossref] [ Google Scholar]
- Li M, Jiang Y, Hou Q, Zhao Y, Zhong L, Fu X. Potential pre-activation strategies for improving therapeutic efficacy of mesenchymal stem cells: current status and future prospects. Stem Cell Research & Therapy 2022; 13(1):146. doi: 10.1186/s13287-022-02822-2 [Crossref] [ Google Scholar]
- Baldari S, Di Rocco G, Piccoli M, Pozzobon M, Muraca M, Toietta G. Challenges and Strategies for Improving the Regenerative Effects of Mesenchymal Stromal Cell-Based Therapies. International Journal of Molecular Sciences 2017; 18(10):2087. doi: 10.3390/ijms18102087 [Crossref] [ Google Scholar]
- Choudhery MS. Strategies to improve regenerative potential of mesenchymal stem cells. World J Stem Cells 2021; 13(12):1845-62. doi: 10.4252/wjsc.v13.i12.1845 [Crossref] [ Google Scholar]
- Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nature Medicine 2003; 9(9):1195-201. doi: 10.1038/nm912 [Crossref] [ Google Scholar]
- Ganji F, Abroun S, Baharvand H, Aghdami N, Ebrahimi M. Differentiation potential of o Bombay human-induced pluripotent stem cells and human embryonic stem cells into fetal erythroid-like cells. Cell J 2015; 16(4):426-39. doi: 10.22074/cellj.2015.489 [Crossref] [ Google Scholar]
- Goldsmith EC, Bradshaw AD, Spinale FG. Cellular mechanisms of tissue fibrosis 2 Contributory pathways leading to myocardial fibrosis: moving beyond collagen expression. Am J Physiol Cell Physiol 2013; 304(5):C393-402. doi: 10.1152/ajpcell.00347.2012 [Crossref] [ Google Scholar]
- Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014; 510(7504):273-7. doi: 10.1038/nature13233 [Crossref] [ Google Scholar]
- Firoozi S, Pahlavan S, Ghanian MH, Rabbani S, Tavakol S, Barekat M. A cell-free SDKP-conjugated self-assembling peptide hydrogel sufficient for improvement of myocardial infarction. Biomolecules 2020; 10(2):205. doi: 10.3390/biom10020205 [Crossref] [ Google Scholar]
- Fonoudi H, Ansari H, Abbasalizadeh S, Rezaei Larijani M, Kiani S, Hashemizadeh S. A universal and robust integrated platform for the scalable production of human cardiomyocytes from pluripotent stem cells. Stem Cells Transl Med 2015; 4(12):1482-94. doi: 10.5966/sctm.2014-0275 [Crossref] [ Google Scholar]
- Rackov G, Garcia-Romero N, Esteban-Rubio S, Carrión-Navarro J, Belda-Iniesta C, Ayuso-Sacido A. vesicle-mediated control of cell function: the role of extracellular matrix and microenvironment. Front Physiol 2018; 9:651. doi: 10.3389/fphys.2018.00651 [Crossref] [ Google Scholar]
- Garrido-Pascual P, Alonso-Varona A, Castro B, Burón M, Palomares T. H2O2-preconditioned human adipose-derived stem cells (HC016) increase their resistance to oxidative stress by overexpressing Nrf2 and bioenergetic adaptation. Stem Cell Res Ther 2020; 11(1):335. doi: 10.1186/s13287-020-01851-z [Crossref] [ Google Scholar]
- Drobiova H, Sindhu S, Ahmad R, Haddad D, Al-Mulla F, Al Madhoun A. Wharton’s jelly mesenchymal stem cells: a concise review of their secretome and prospective clinical applications. Front Cell Dev Biol 2023; 11:1211217. doi: 10.3389/fcell.2023.1211217 [Crossref] [ Google Scholar]
- Hao C, Lu Z, Zhao Y, Chen Z, Shen C, Ma G. Overexpression of GATA4 enhances the antiapoptotic effect of exosomes secreted from cardiac colony-forming unit fibroblasts via miRNA221-mediated targeting of the PTEN/PI3K/AKT signaling pathway. Stem Cell Res Ther 2020; 11(1):251. doi: 10.1186/s13287-020-01759-8 [Crossref] [ Google Scholar]
- Cantoni S, Cavallini C, Bianchi F, Bonavita F, Vaccari V, Olivi E. Rosuvastatin elicits KDR-dependent vasculogenic response of human placental stem cells through PI3K/AKT pathway. Pharmacol Res 2012; 65(3):275-84. doi: 10.1016/j.phrs.2011.12.004 [Crossref] [ Google Scholar]
- Shabbir A, Zisa D, Lin H, Mastri M, Roloff G, Suzuki G. Activation of host tissue trophic factors through JAK-STAT3 signaling: a mechanism of mesenchymal stem cell-mediated cardiac repair. Am J Physiol Heart Circ Physiol 2010; 299(5):H1428-38. doi: 10.1152/ajpheart.00488.2010 [Crossref] [ Google Scholar]
- Liu ZL, Chen HH, Zheng LL, Sun LP, Shi L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct Target Ther 2023; 8(1):198. doi: 10.1038/s41392-023-01460-1 [Crossref] [ Google Scholar]
- Zakikhan K, Pournasr B, Vosough M, Nassiri-Asl M. In vitro generated hepatocyte-like cells: a novel tool in regenerative medicine and drug discovery. Cell J 2017; 19(2):204-17. doi: 10.22074/cellj.2016.4362 [Crossref] [ Google Scholar]